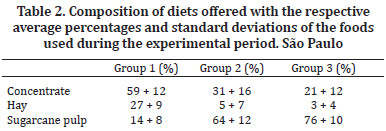Abstracts
To evaluate the influence of diets with different degrees of energy deficiency on the hormonal profile and vital functions, 12 steers were randomly distributed into 3 groups of 4 animals. For 140 days, each group received (G1) a diet to promote a weight gain of 900gr/day (17.7 Mcal/d DE and 13% CP), (G2) 80% of the maintenance requirements (5.8 Mcal/d DE and 7% CP), or (G3) 60% of the maintenance requirements (4.7 Mcal/d DE and 5% CP). In G2 and G3, the energy deficit caused a marked decrease in the heart rate and respiratory rate and a reduction in the blood levels of Insulin like growth factor-1 (IGF-1) and triiodothyronine (T3). The decrease in heart rate, respiratory movement and, to a lesser extent, reduction of the rectal temperature, reflected the low status of energy and was negatively impacted by the low levels of T3. There was a strong correlation between the hormones T3 and IGF-1 (r=0.833). There were also strong correlations between T3 and HR (r=0.701), T3 and RR (r=0.632), IGF-1 and HR (r=0.731), and IGF-1 and RR (r=0.679). There were intermediate correlations between T3 and TºC (r=0.484), T3 and insulin (r=0.506), IGF-1 and insulin (r=0.517), and IGF-1 and TºC (r=0.548). This study showed the influence of a long period of providing an energy-deficient diet on animal performance, correlating hormonal status and vital functions in growing cattle. The results indicated that the evaluated parameters represent an important tool for the early detection of dietary deficiency.
Cattle; vital functions; IGF-1; ruminants; T3
Para avaliar a influência de dietas deficientes de energia sobre o perfil hormonal e as funções vitais em bovinos, 12 garrotes foram aleatoriamente distribuídos em três grupos com quatro animais para receber por 140 dias, as seguintes dietas: (G1) adequada, para ganho de peso de 900g/dia (17,7 Mcal/d de ED e 13% de PB); (G2) 80% dos requerimentos de mantença (5,8 Mcal/d de ED e 7% de PB); e (G3) 60% desses requerimentos (4,7 Mcal/d de ED e 5% de PB). O déficit energético provocou nos grupos G2 e G3 acentuada diminuição da frequência cardíaca e da frequência respiratória além da redução nos teores sanguíneos de IGF-1 e T3. A diminuição do número de batimentos cardíacos, movimentos respiratórios e em menor grau a queda na temperatura retal refletiram o baixo status energético imprimido e foram influenciados negativamente pelos baixos teores de T3. Houve correlação de alta intensidade entre os hormônios T3 e IGF-1 (r=0,833). As correlações entre os hormônios T3 e IGF-1 e as funções vital exibiram altas e médias intensidades. Houve elevada correlação entre T3 e FC (r=0,701), T3 e FR (r=0,632), IGF-1 e FC (r=0,731), IGF-1 e FC (r=0,679). Houve média correlação entre T3 e TºC (r=0,484), T3 e insulina (r=0,506), IGF-1 e insulina (r=0,517) e IGF-1 e TºC (r=0,548). O presente trabalho apontou a influência do longo período do oferecimento de dietas deficientes em energia sobre o desempenho animal correlacionando o perfil hormonal e as funções vitais nos bovinos em crescimento. Os resultados indicaram que os parâmetros avaliados podem ser uma ferramenta importante na detecção precoce da carência nutricional.
Bovinos; funções vitais; IGF-1; ruminantes; T3
ANIMAL MORPHOPHYSIOLOGY
Long term dietary deficiency in steers: vital functions and T3 and IGF-1 relationships
Carência alimentar prolongada em garrotes: funções vitais e suas relações com os teores de T3 e IGF-1
Alessandra S. Lima; Maria Claudia A. Sucupira* * Corresponding author: msucupir@usp.br ; Enrico L. Ortolani
Departamento de Clínica Médica, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo (USP), Av. Prof. Dr. Orlando Marques de Paiva 87, Cidade Universitária, São Paulo, SP 05508-270, Brazil. E0-mails: alessandralima@usp.br, msucupir@usp.br, ortolani@usp.br
ABSTRACT
To evaluate the influence of diets with different degrees of energy deficiency on the hormonal profile and vital functions, 12 steers were randomly distributed into 3 groups of 4 animals. For 140 days, each group received (G1) a diet to promote a weight gain of 900gr/day (17.7 Mcal/d DE and 13% CP), (G2) 80% of the maintenance requirements (5.8 Mcal/d DE and 7% CP), or (G3) 60% of the maintenance requirements (4.7 Mcal/d DE and 5% CP). In G2 and G3, the energy deficit caused a marked decrease in the heart rate and respiratory rate and a reduction in the blood levels of Insulin like growth factor-1 (IGF-1) and triiodothyronine (T3). The decrease in heart rate, respiratory movement and, to a lesser extent, reduction of the rectal temperature, reflected the low status of energy and was negatively impacted by the low levels of T3. There was a strong correlation between the hormones T3 and IGF-1 (r=0.833). There were also strong correlations between T3 and HR (r=0.701), T3 and RR (r=0.632), IGF-1 and HR (r=0.731), and IGF-1 and RR (r=0.679). There were intermediate correlations between T3 and TºC (r=0.484), T3 and insulin (r=0.506), IGF-1 and insulin (r=0.517), and IGF-1 and TºC (r=0.548). This study showed the influence of a long period of providing an energy-deficient diet on animal performance, correlating hormonal status and vital functions in growing cattle. The results indicated that the evaluated parameters represent an important tool for the early detection of dietary deficiency.
Index terms: Cattle, vital functions. IGF-1, ruminants, T3.
RESUMO
Para avaliar a influência de dietas deficientes de energia sobre o perfil hormonal e as funções vitais em bovinos, 12 garrotes foram aleatoriamente distribuídos em três grupos com quatro animais para receber por 140 dias, as seguintes dietas: (G1) adequada, para ganho de peso de 900g/dia (17,7 Mcal/d de ED e 13% de PB); (G2) 80% dos requerimentos de mantença (5,8 Mcal/d de ED e 7% de PB); e (G3) 60% desses requerimentos (4,7 Mcal/d de ED e 5% de PB). O déficit energético provocou nos grupos G2 e G3 acentuada diminuição da frequência cardíaca e da frequência respiratória além da redução nos teores sanguíneos de IGF-1 e T3. A diminuição do número de batimentos cardíacos, movimentos respiratórios e em menor grau a queda na temperatura retal refletiram o baixo status energético imprimido e foram influenciados negativamente pelos baixos teores de T3.
Houve correlação de alta intensidade entre os hormônios T3 e IGF-1 (r=0,833). As correlações entre os hormônios T3 e IGF-1 e as funções vital exibiram altas e médias intensidades. Houve elevada correlação entre T3 e FC (r=0,701), T3 e FR (r=0,632), IGF-1 e FC (r=0,731), IGF-1 e FC (r=0,679). Houve média correlação entre T3 e TºC (r=0,484), T3 e insulina (r=0,506), IGF-1 e insulina (r=0,517) e IGF-1 e TºC (r=0,548). O presente trabalho apontou a influência do longo período do oferecimento de dietas deficientes em energia sobre o desempenho animal correlacionando o perfil hormonal e as funções vitais nos bovinos em crescimento. Os resultados indicaram que os parâmetros avaliados podem ser uma ferramenta importante na detecção precoce da carência nutricional.
Termos de indexação: Bovinos, funções vitais, IGF-1, ruminantes, T3.
INTRODUCTION
Cattle that are raised under extensive conditions and that feed primarily on tropical grasses suffer during the dry season when the production of grass is diminished and drastic changes in the chemical composition of the diet occur. The sharp drop in crude protein and energy and the increase in the levels of crude fiber decrease the digestibility, leading to lower productivity and loss (González 2000). In Brazil, among the 186.7 million beef cattle, more than 98% are raised in extensive conditions (IBGE 2010).
The physiological response to a lower energy intake triggers a series of reactions and consequences that allow the organism to better adapt to the energy deficit. These changes have important consequences on both the hormonal profile and the animal performance (Lima et al. 2011).
Endocrine and metabolic responses to undernutrition are primarily aimed at maintaining, within certain limits, the constancy of the internal environment (homeostasis). Thus, short-, medium and long-term adaptations in underfed animals result in the orderly mobilization of endogenous substrates (body reserves), sparing glucose and amino acids, while lowering the metabolic rate and energy expenditure (Chilliard et al. 2000).
The vital functions, which rely on the basal metabolism, were studied by Oetzel (1988), who found that animals subjected to protein-energy malnutrition generally have a decreased rectal temperature, even if the animals are kept stabled or at an ambient temperature. Koeppen & Stanton (2009) described both a decrease in the heart rate and a decrease in the respiratory rate in humans over a period of stress, fasting or deficient diet as the concentration of thyroid hormones decreased. Although it has been suggested in some species, the decrease in the heart rate has not been studied in cattle subjected to a reduced energy intake.
Ruminants have the ability to adapt to periods of malnutrition because they can reduce their maintenance requirements by reducing the basal metabolic rate, an effect that is mediated by lower concentrations of thyroid hormones (Oetzel 1988, Chilliard et al. 1998, Gómez-Pastén et al 1999). Thyroid hormones play a key role in the basal metabolism. The general effects of these hormones in the body include the stimulation of protein synthesis, glycolysis, gluconeogenesis and intestinal absorption of glucose; the stimulation of cardio-circulatory activity by increasing the heart rate, cardiac output and blood flow; the stimulation of neuronal development; and increased transmission in neural cells (Capen & Martin 2003). Although the hormone thyroxine (T4) is secreted in greater amounts by the thyroid gland, triiodothyronine (T3) is the biologically active form in the body (Koeppen & Stanton 2009), and it must therefore be measured in studies of energy metabolism, especially in cases of food restriction.
Various hormones regulate the growth and the utilization of certain nutrients, in particular energy. IGF-1 (Insulin-Like Growth Factor-1) plays an important role in mediating growth, development and tissue differentiation, particularly the increase in protein synthesis. Insulin is an important anabolic hormone that plays a significant role in regulating numerous metabolic pathways, increasing glucose uptake by stimulating its storage as glycogen and its use as a substrate for lipogenesis (Koeppen & Stanton 2009).
This study was conducted to evaluate the influence of the effects of long-term exposure of cattle to energy-deficient diets on animal performance with respect to hormonal status and vital functions in growing cattle.
MATERIALS AND METHODS
The present study used 12 yearling crossbred male steers that were healthy, aged approximately 10 months and had an average weight of 165 kg at the beginning of the experiment. The animals were kept in individual pens where there was the possibility for evaluating the individual food consumption.
The experimental period was 140 days (divided into 10 periods of 14 days). The 12 steers were randomly distributed into three experimental groups that received diets that differed in the proportion of nutrients and were formulated according to the tables described in nutritional NRC (1984) for cattle. The animals in Group 1 received a diet rich in protein and energy, sufficient to support a gain of 900g/day - 17.7 Mcal/day of digestible energy (DE) and 13% crude protein (CP). Group 2 received an amount of protein sufficient to gain 100 g/day and an energy level that was 20% below the maintenance requirement - 5.8 Mcal/day of digestible energy (DE) and 7% crude protein (CP). Group 3 received a diet with sufficient protein for the maintenance of the animals and an energy level that was 40% below the maintenance requirement - 4.7 Mcal/day of digestible energy (DE) and 5% crude protein (CP). The diet for all three groups of animals was composed of dehydrated crushed sugarcane, coast-cross hay (Cynodon dactylon) and commercial concentrate with 22% crude protein (CP) (Table 1).
The diets were formulated using varying amounts of food (Table 2). The amount of dry matter (DM) of the feed offered was equivalent to 2.75% of the body weight (BW). Mineral salt and water were provided ad libitum during the experimental period.
The main vital functions (Heart Rate - HR, Respiratory Rate - RR, and Rectal Temperature - TºC) were measured at 14 days just before the collection of blood samples. The blood samples were always collected three hours after feeding the steers. The concentrations of IGF-1 in the plasma of the bovines were quantified by an immunoradiometric assay (IRMA) after extraction using the kit DSL-5600 (Diagnostic Systems Laboratories, Inc.). The plasma concentration of T3 was determined with the kit DPC/USA 35 TKT (Kit Coat-A-Count T3 Total). The plasma concentration of insulin was determined with the kit DPC/USA 35 TKIN5 (Kit Coat-A-Count Insulin).
The normal distribution of the data was verified by the Kolmogorov-Smirnov test. To assess the differences between the means of the results, an analysis of variance (one-way ANOVA) and the Tukey's test were performed for data exhibiting a parametric distribution. When the data exhibited a nonparametric distribution, they were transformed into log (x+1). Significance was established when P<0.05 for all tests. The data are expressed as the mean (± standard deviation). The correlation among the variables was investigated by the means of the Pearson correlation coefficient. The correlation was rated high when r>0.6, intermediate when r ranged from 0.3 to 0.6 and low when r<0.3. MINITAB® software version 14.1 (GlobalTech InformáticaTM, Belo Horizonte, Minas Gerais, Brazil) was used for statistical analysis.
The present study was approved by the Bioethics Committee of the School of Veterinary Medicine and Zootechnics of the University of São Paulo (Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo - FMVZ-USP) under protocol number 595/2004.
RESULTS
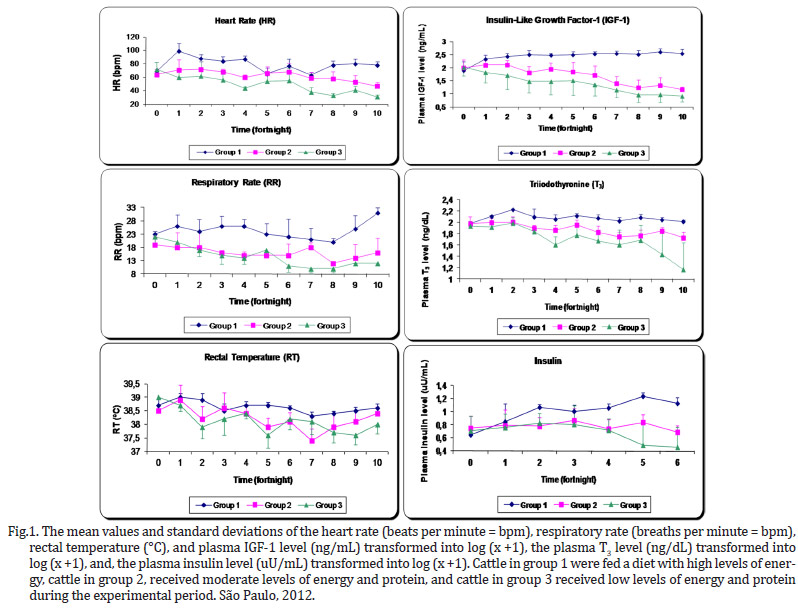
The dietary energy restrictions prolonged changes in hormonal levels (plasma concentrations of IGF-1, T3 and insulin) and in the vital functions, including the heart rate (beats per minute - BPM), respiratory rate (breaths per minute - BPM) and rectal temperature (ºC) in the animals (Fig.1). The animals belonging to the groups that were exposed to severe energy restriction showed prominent decreases in the measured parameters.
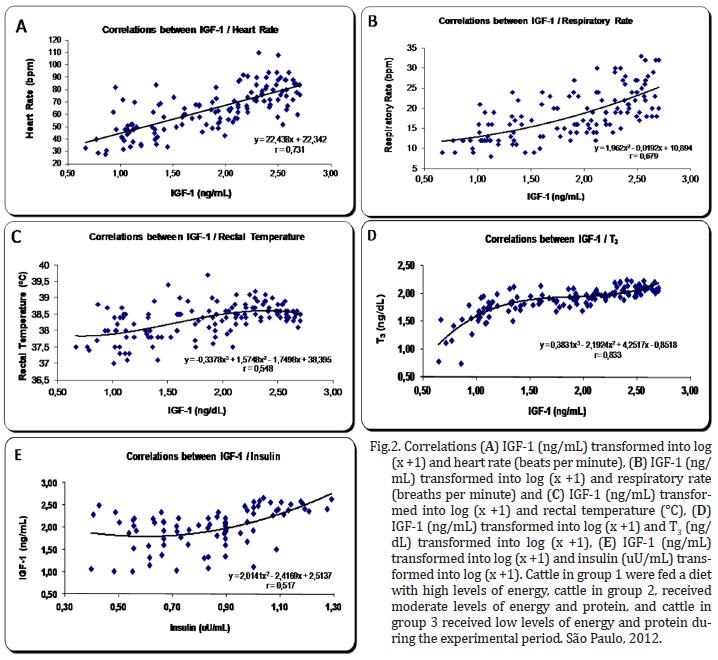
There was a high correlation between the hormones T3 and IGF-1 r=0,833 (P=0,0001). The correlations between the variables of hormones T3 and IGF-1 and the vital functions exhibited high and intermediate intensity.
There were strong correlations between T3 and HR r=0.701 (P=0.0001), T3 and RR r=0.632 (P=0.001), IGF-1 and HR r=0.731 (P=0.0001), and IGF-1 and RR r=0.679 (P=0.001).
There were intermediate correlations between IGF-1 and TºC r=0.548 (P=0.001), IGF-1 and insulin r=0.517 (P=0.0001), T3 and insulin r=0.506 (P=0.001), and T3 and TºC r=0.484 (P=0.001) (Fig.2 and 3 ).
DISCUSSION
In situations of deficiency, protein and energy needs are supplied to the body through the mobilization of nutrients from the tissues, resulting in a loss of body condition and weight (Ensminger 1988, Sucupira 2003). To conserve energy, these changes also significantly affect the vital functions and hormone profile.
As the degree of energy malnutrition increased in the animals in this study, both the heart rate and breathing became significantly lower, however, this was not the case for the rectal temperature (Fig.1). In addition to these changes, the malnourished animals showed apathy, weakness and decreased physical activity. While the control group had a heartbeat within the normal range of 60 to 80 BPM, at the end of 140 days, the animals in group 3 exhibited a reduction to 31 BPM, which is 57% below the average baseline. In their work with foals aged 6 months and fed diets with energy restriction, Ellis & Lawrence (1978) observed a decrease in the basal metabolism expressed clearly by the 38% decrease in heart rate during the 147-day experiment.
Energy malnutrition was also shown to cause this change in other species of mammals, such as humans and horses (Ellis & Lawrence 1978, Koeppen & Stanton 2009). Several hormones directly or indirectly regulate the heart rate in animals, especially the catecholamines, which have positive chronotropic action. Studies with steers subjected to energy malnutrition showed a decrease in plasma norepinephrine (Koeppen & Stanton 2009). However, this reduction is modulated by the action of triiodothyronine (T3), which is also reduced in malnourished animals, as was demonstrated in this experiment. The reduction of T3 induces a lower secretion and potentiation of norepinephrine and epinephrine. Furthermore, both T3 and thyroxine (T4) have direct effects on the heart, increasing chronotropism and inotropism. In this experiment, we did not determine the plasma concentrations of norepinephrine or epinephrine, but we demonstrated that there was a high positive correlation (r=0.701) between the concentrations of T3 and heart rate (Fig.3 ).
In parallel, malnutrition caused a drop in the respiratory rate below the reference values from 20 to 30 BPM (Radostits et al 2007), but this decline was not as sharp as the drop in the heart rate, which was to 45% of the baseline value. When we evaluated the change in respiratory rate over time (Fig.1), it appears that a significant decrease (P<0.0001) relative to the baseline was evident in group 3 by the 2nd fortnight, and had further declined by the 6th fortnight. This trend continued until the end of the study. These findings are consistent with the concepts published by Chilliard et al. (1998) on the development of malnutrition, energy expenditure and oxygen consumption by the body. According to these authors, these changes develop in three distinct phases. In the short term, there is a slight decrease in these variables. As early as the 2nd and 3rd week, there is a clear reduction due to the sharp drop in metabolism and a reduction in liver weight and other gastrointestinal organs. Later, these metabolic activities are reduced slowly and gradually. In other words, the respiratory rate reflects the decrease in oxygen consumption by the malnourished animals.
The respiratory rate is considered by Swenson and Reece (1996) to be an excellent indicator of health, but they note that this variable may be affected by body size, age, physical activity, excitement, ambient temperature, pregnancy, degree of filling of the digestive tract and health status. Regarding the hormonal influence, it is noteworthy that the decreased levels of catecholamines and thyroid hormones also negatively influence the respiratory rate (Koeppen & Stanton 2009). In our study, there was a positive correlation (r=0.632) between the respiratory rate and the levels of T3 (Fig. 3 ).
There were also strong correlations between IGF-1 and heart rate (r = 0.731) and respiratory rate (r = 0.679) (Fig. 2). Thus far, there are no studies indicating that IGF-1 has a direct influence on the control of these variables.
In cases of malnourishment, hypothermia is most likely due to the decreased basal metabolic rate and continued loss of thermal body insulation (Herdt 1988). The overall mean rectal temperature was slightly lower in animals in the energy-restricted groups, 0.4ºC below the control group, reaching a value of 38.2±0.5ºC. Yet this average falls within the expected value for animals of this age (37.8-39.2ºC) (Radostits et al. 2007). However, one animal in group 3 presented a low temperature of 37.4ºC at the peak of the deficiency. In comparison to the heart and respiratory rates, the body temperature is much more critical for the survival of the animal, and thus, it is maintained as long as possible.
Although there is a decrease in the metabolism of many organs (splanchnic tissue) and muscles during malnutrition, the overall energy expenditure of cattle is even greater in relation to the metabolic weight (Chilliard et al. 1998). The maintenance of the basal temperature, reflected here by the rectal temperature, is fundamental to survival as intracellular enzymes only function within a strict temperature range in warm-blooded animals (Nelson & Cox 2011). The maintenance of the basal body temperature in cases of malnourished animals can be explained by the operation of various enzymes. Erythrocytes, the liver and other vital organs are conditioned to function within a narrow temperature range in homeothermic animals (Nelson & Cox 2011). These findings conflict with the results of Oetzel (1988) and Radostits et al. (2007), who showed that undernourished ruminants undergo a substantial drop in the rectal temperature (32.2-36.7ºC). Although these authors do not cite references supporting this claim, it is believed that these data were obtained from animals in an advanced state of cachexia. This experiment indicates that hypothermia does not occur under all conditions of undernourishment.
There was a moderate positive correlation (r=0.484) between the levels of T3 and the rectal temperature (Fig.3 ). Undoubtedly, this thyroid hormone controls the expenditure of energy, heat production and oxygen consumption. Although a moderate positive correlation existed (r=0.548) between IGF-1 and rectal temperature, this relationship has not been adequately described in the literature (Fig.2). It is expected that well-nourished animals with high metabolic rates have rectal temperatures that are slightly higher than malnourished animals. The IGF-1 directly reflects the metabolic rate, but the question remains as to whether this hormone has a direct influence on the rectal temperature, a minor effect, or merely reflects the action of T3, as the correlation between these two hormones was also high (r=0.833) (Fig.2).
The concentration of IGF-1 was the best indicator of the energy status with a significant difference between the energy deficient groups and the control (Fig.1). This variable also proved to be sensitive to the low coefficient of variation. This is the main hormone of the group known as somatomedins, which are produced in the liver in response to growth hormone (GH). Several tissues have receptors for IGF-1, particularly the bony cartilaginous epiphysis, the mammary gland and skeletal muscles. In muscle, IGF-1 mainly has a hypertrophic effect and, to a lesser extent, a hyperplastic effect, influencing amino acid uptake and protein synthesis by cells in skeletal muscle (Lawrance & Fowler 1997, Squires 2003).
T3 appears to diminish the expression of mRNA for IGF modulated and controlled by HC (Hynes et al. 1987). This seems to occur mainly in states of mild or marked hyperthyroidism in which the excess T3 causes reduced production of IGF-1, promoting a decrease in muscle protein synthesis (Elsasser et al. 1993). Still, the study indicated that there was a positive relationship between IGF-1 and T3, as the nutritional quality offered to calves increased from 160 to 240 kg, particularly for the intake of protein, under conditions reasonably similar to those found in the control group of this experiment (Gerrits et al. 1998). These results demonstrate that under natural conditions of normal thyroid levels, there is a significant positive correlation between T3 and IGF-1 (r=0.833) (Fig.2). Lima et al. 2011 found a positive correlation between IGF-1 and body weight (r=0.857) and dry matter intake (r=0.885). Insulin somehow regulates the ability of GH to stimulate the production of IGF-1 by hepatocytes (Lawrance & Fowler 1997). In the present study, the positive intermediate correlation between IGF-1 and insulin was r=0.517.
Energy restriction has a direct influence on T3, and there was a clear difference between the control group and the energy restricted groups by the 6th period of experimentation (Fig.1). This finding indicates that there was less biotransformation of T4 to T3, which is usually mediated by type I and II 5'deiodinase enzymes. This biotransformation occurs mostly in other organs, not in the thyroid, resulting in high blood flow and rapid shifts in plasma, especially in the liver tissue. The activity of these enzymes is controlled by the amount of energy available in the organs, reflecting the nutritional status of animals (Gerrits et al. 1998). A minor part of the synthesis of T3 requires the amino acid tyrosine. A molecule of tyrosine containing an iodine atom (monoiodotyrosine - MIT) combines with a similar molecule of tyrosine with two iodines (diiodotyrosine - DIT). The combination of DIT and MIT results in triiodothyronine, also referred to as T3. The MIT and DIT are derived from a glycoprotein complex called thyroglobulin, which is essential for the synthesis of thyroid hormones.
Both insulin and IGF-1 are required for the synthesis of thyroglobulin. In cases of malnutrition, the synthesis of thyroglobulin will decrease due to the lower actuation of insulin and IGF-1, which are also influenced by diet (Koeppen & Stanton 2009). In fact, in the present study, a strong correlation was found between T3 and IGF-1 (r=0.833), and an intermediate correlation was found with insulin (r=0.506). Oetzel (1988) stated that animals subjected to malnutrition show a significant decrease in the concentration of insulin and in the amounts of thyroid hormones, consistent with this study.
A close relationship between the thyroid hormones and growth promoters in animals accompanied by an increase in the general metabolism has been described (Squires 2003). This relationship was also demonstrated by Lima et al. 2011, who found a positive correlation between T3 and body weight (r=0.710) and increased food consumption (r=0.732).
In general, the restriction of energy in group 3 resulted in lower values compared with group 2 for the following variables: heart rate, IGF-1 and T3. Thus, these variables showed a sensitivity and responsiveness reflecting effects that were dependent on the level of deprivation.
CONCLUSION
The results indicate that prolonged energy deficiency in growing cattle results in a sharp decrease in heart rate and hormonal levels as well as a reduction in blood levels of IGF-1 and T3. In the current study, the determination of these parameters may be an important tool for the early detection of disabilities.
Received on December 13, 2013.
Accepted for publication on July 27, 2014.
- Capen C.C. & Martin S.L. 2003. The thyroid gland, p.35-70. In: Pineda M.H. & Dooley M.P. (Eds), McDonald's Veterinary Endocrinology and Reproduction. Iowa State Press, Ames. 597p.
- Chilliard Y., Bocquier F. & Doreall M. 1998. Digestive and metabolic adaptation of ruminants to undernutrition and consequences on reproduction. Reprod. Nutr. Development 38(2):131-152.
- Chilliard Y., Ferlay A., Faulconnier Y., Bonnet M., Rouel J. & Bocquier F. 2000. Adipose tissue metabolism and its role in adaptations to undernutrition in ruminants. Proc. Nutr. Soc. 59(1):127-134.
- Ellis R.N.W. & Lawrence T.L.J. 1978. Energy under-nutrition in the weanling filly foal. III. Effects on heart rate and subsequent voluntary food intake. Brit. Vet. J. 134 (4):333-341.
- Elsasser T.H., Rumsey T.S. & Kahl S. 1993. Relationship between the thyroid and somatotropic axes in steers. 2. Effects of thyroid status on plasma concentrations of insulin-like growth factor I (IGF-1) and the IGF-1 response to growth hormone. Domest. Anim. Endocrinol. 10(2):71-85.
- Ensminger M.E. 1987. Feeding beef cattle, p.239-348. In: Ensminger M.E. (Ed.), Beef Cattle Science. 6th ed. The Interstate, Danville, Illinois. 1030p.
- Gerrits W.J.J., Decuypere E., Verstegen M.W.A. & Karabinas V. 1998. Effect of protein-free energy intake on plasma concentrations of insulin-like growth factor I and thyroid hormones in preruminant calves. J. Anim. Sci. 76:1356-1363.
- Gómez-Pastén M., Mora O., Pedraza-Chaverri J. & Simada A. 1999. The effect of a long term feed restrition on metabolism and tissue composition of goats. J. Agricult. Sci., Cambridge, 132:227-232.
- González F.H.D. 2000. Uso do perfil metabólico para determinar o status nutricional em gado de corte, p.63-74. In: González F.H.D., Barcellos J.O., Ospina H. & Ribeiro L.A.O. (Eds), Perfil Metabólico em Ruminantes: seu uso em nutrição e doenças nutricionais. Gráfica da Universidade Federal do Rio Grande do Sul, Porto Alegre. 108p.
- Herdt H.H. 1988. Fuel homeostasis in the ruminant. Vet. Clin. North Am., Food Anim. Pract. 4(2):213-231.
- Hynes M.A., Van Wyk J.J., Brooks P.J., D'ercole A.J., Jansen M. & Lund P.K. 1987. Growth hormone dependence of somatomedin-C/insulin-like growth factor I and insulin-like growth factor II nRNA. Mol. Endocrinol. 1:233-242.
- IBGE 2010. Produção da Pecuária Municipal. Instituto Brasileiro de Geografia e Estatística, Brasília, DF.
- Koeppen B.M. & Stanton B.A. 2009. Berne and Levy's Fisiologia. 6th ed. Elsevier, Rio de Janeiro. 864p.
- Lawrence T.L.J. & Fowler V.R. 1997. Growth of Farm Animals. 2nd ed. CAB International, New York. 330p.
- Lima A.S., Sucupira M.C.A. & Ortolani E.L. 2011. Bovinos submetidos a dietas deficientes em energia por longo período: desempenho animal e sua relação com os teores de T3 e IGF-1. Braz. J. Vet. Res. Anim. Sci. 48(1):19-26.
- Nelson D.L. & Cox M.M. 2011. Princípios de Bioquímica de Lehninger. 5th ed. Editora Artmed, Porto Alegre. 1304p.
- NRC 1984. Nutrient Requeriments of Beef Cattle. 6th ed. Committe on Animal Nutrition, National Research Council. National Academy of Science, Washingyon, DC. 90p.
- OetzeL G.R. 1988. Protein energy malnutrition in ruminants. Vet. Clin. North Am., Food Anim. Pract. 4(2):317-329.
- Radostits O.M., Gay C.C., Hinchcliff K.W. & Constable P.D. 2007. Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th ed. W.B. Saunders, Baltimore. 2065p.
- Squires, E.J. 2003. Applied Animal Endocrinology. CABI Publishing, Massachusetts. 234p.
- Sucupira M.C.A. 2003. Estudo comparativo de exames clínico-laboratoriais no diagnóstico de carência energética prolongada em garrotes. Tese de Doutorado em Clínica Médica, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, SP. 173p.
- Swenson M.J. & Reece W.O. 1996. Duke's Fisiologia dos Animais Domésticos. 11ª ed. Guanabara Koogan, Rio de Janeiro. 856p.
Publication Dates
-
Publication in this collection
25 Nov 2014 -
Date of issue
Sept 2014
History
-
Accepted
27 July 2014 -
Received
13 Dec 2013