Abstract
Immune response plays an important role in the development of hepatic fibrosis. In the present study, we investigated the effects of quercetin on hepatitis and hepatic fibrosis induced by immunological mechanism. In the acute hepatitis model, quercetin (2.5 mg/kg) was injected iv into mice 30 min after concanavalin A (Con A) challenge. Mice were sacrificed 4 or 24 h after Con A injection, and aminotransferase tests and histopathological sections were performed. Treatment with quercetin significantly decreased the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Consistent with this observation, treatment with quercetin markedly attenuated the pathologic changes in the liver. A hepatic fibrosis model was also generated in mice by Con A challenge once a week for 6 consecutive weeks. Mice in the experimental group were treated with daily iv injections of quercetin (0.5 mg/kg). Histopathological analyses revealed that treatment with quercetin markedly decreased collagen deposition, pseudolobuli development, and hepatic stellate cells activation. We also examined the effects of quercetin on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor beta (TGF-β) pathways by immunohistochemistry and real-time reverse transcriptase-polymerase chain reaction (RT-PCR). NF-κB and TGF-β production was decreased after treatment with quercetin, indicating that the antifibrotic effect of quercetin is associated with its ability to modulate NF-κB and TGF-β production. These results suggest that quercetin may be an effective therapeutic strategy in the treatment of patients with liver damage and fibrosis.
Quercetin; Concanavalin A; Acute hepatitis; Hepatic fibrosis; Liposome
Introduction
Hepatic fibrosis is a common response to liver injury caused by various etiologies (viral, toxic, metabolic, and autoimmune) that ultimately lead to cirrhosis and related complications (such as portal hypertension, liver failure, and hepatocellular carcinoma) characterized by increased deposition of extracellular matrix (ECM) (11. Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 2005; 45: 605-628, doi: 10.1146/annurev.pharmtox.45.120403.095906.
https://doi.org/10.1146/annurev.pharmtox...
). Liver fibrosis is a major health problem worldwide. It is estimated that up to 1% of the populations might have histological cirrhosis (22. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008; 371: 838-851, doi: 10.1016/S0140-6736(08)60383-9.
https://doi.org/10.1016/S0140-6736(08)60...
). To date, there are however few therapeutic strategies for the treatment of patients with liver fibrosis and liver cirrhosis (33. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010; 7: 425-436, doi: 10.1038/nrgastro.2010.97.
https://doi.org/10.1038/nrgastro.2010.97...
).
It has been shown that the immune response plays an important role in the development of hepatic fibrosis (11. Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 2005; 45: 605-628, doi: 10.1146/annurev.pharmtox.45.120403.095906.
https://doi.org/10.1146/annurev.pharmtox...
,33. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010; 7: 425-436, doi: 10.1038/nrgastro.2010.97.
https://doi.org/10.1038/nrgastro.2010.97...
). Interaction among hepatic stellate cells (HSCs), macrophages, and CD4 T cells (Th2) occurs within a microenvironment of growth factors and cytokines, which activate HSCs, possibly leading to the production of ECM and subsequently to liver fibrosis (44. Tanaka H, Leung PS, Kenny TP, Gershwin ME, Bowlus CL. Immunological orchestration of liver fibrosis. Clin Rev Allergy Immunol 2012; 43: 220-229, doi: 10.1007/s12016-012-8323-1.
https://doi.org/10.1007/s12016-012-8323-...
). Moreover, it has been reported that repeated flares or continuous recruitment of inflammatory cells to the liver ultimately results in fibrosis, cirrhosis, and possible hepatocellular carcinoma (55. Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000; 275: 2247-2250, doi: 10.1074/jbc.275.4.2247.
https://doi.org/10.1074/jbc.275.4.2247...
). Hepatic fibrosis induced by administration of concanavalin A (Con A) was reported to be caused by continuous cellular injury after immune response without direct hepatotoxic activity, resembling chronic hepatitis in humans (66. Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, et al. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol 1999; 11: 1491-1500, doi: 10.1093/intimm/11.9.1491.
https://doi.org/10.1093/intimm/11.9.1491...
).
The flavonoid quercetin (3,3,4,5,7-pentahydroxy flavone) has shown anti-inflammatory and anti-fibrotic effects such as experimental allergic encephalomyelitis, airways allergic inflammatory model, and ultraviolet (UV)-irradiated primary human keratinocytes (77. Valerio DA, Georgetti SR, Magro DA, Casagrande R, Cunha TM, Vicentini FT, et al. Quercetin reduces inflammatory pain: inhibition of oxidative stress and cytokine production. J Nat Prod 2009; 72: 1975-1979, doi: 10.1021/np900259y.
https://doi.org/10.1021/np900259y...
8. Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LF, Lemos-Senna E, et al. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res 2010; 61: 288-297, doi: 10.1016/j.phrs.2009.10.005.
https://doi.org/10.1016/j.phrs.2009.10.0...
-99. Vicentini FT, He T, Shao Y, Fonseca MJ, Verri WA Jr, Fisher GJ, et al. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J Dermatol Sci 2011; 61: 162-168, doi: 10.1016/j.jdermsci.2011.01.002.
https://doi.org/10.1016/j.jdermsci.2011....
) in vivo and in vitro. Moreover, our previous study reported that quercetin could inhibit pulmonary fibrosis (1010. Baowen Q, Yulin Z, Xin W, Wenjing X, Hao Z, Zhizhi C, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol 2010; 642: 134-139, doi: 10.1016/j.ejphar.2010.05.019.
https://doi.org/10.1016/j.ejphar.2010.05...
). The effects of quercetin on liver damage induced by carbon tetrachloride, hepatotoxins, or bile duct obstruction have been previously studied (1111. Pavanato A, Tunon MJ, Sanchez-Campos S, Marroni CA, Llesuy S, Gonzalez-Gallego J, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci 2003; 48: 824-829, doi: 10.1023/A:1022869716643.
https://doi.org/10.1023/A:1022869716643...
12. de David C, Rodrigues G, Bona S, Meurer L, Gonzalez-Gallego J, Tunon MJ, et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol 2011; 39: 949-957, doi: 10.1177/0192623311418680.
https://doi.org/10.1177/0192623311418680...
13. Kanter M. Protective effect of quercetin on liver damage induced by chronic toluene exposure in rats. Toxicol Ind Health 2012; 28: 483-491, doi: 10.1177/0748233711414613.
https://doi.org/10.1177/0748233711414613...
-1414. Peres W, Tunon MJ, Collado PS, Herrmann S, Marroni N, Gonzalez-Gallego J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol 2000; 33: 742-750, doi: 10.1016/S0168-8278(00)80305-0.
https://doi.org/10.1016/S0168-8278(00)80...
). However, the effect of quercetin on liver damage based on immunological mechanisms is not yet known. In the present study, we investigated the effects of liposomal quercetin on hepatitis and hepatic fibrosis induced by Con A.
Material and Methods
Experimental animals
Female BALB/c mice (6-8 weeks; 18-20 g) were obtained from the Sichuan University Laboratory Animal Center. The experimental procedures were approved by the Ethics Committees of State Key Laboratory of Biotherapy, West China Hospital, West China Medical School, Sichuan University.
Preparation of liposomal quercetin
Liposomal quercetin was prepared as previously described (1010. Baowen Q, Yulin Z, Xin W, Wenjing X, Hao Z, Zhizhi C, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol 2010; 642: 134-139, doi: 10.1016/j.ejphar.2010.05.019.
https://doi.org/10.1016/j.ejphar.2010.05...
,1515. Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res 2006; 12: 3193-3199, doi: 10.1158/1078-0432.CCR-05-2365.
https://doi.org/10.1158/1078-0432.CCR-05...
). Briefly, lecithin, cholesterol, polyethylene glycol (PEG) 4000 and quercetin (weight ratios: 13:4:1:6) were dissolved in chloroform/methanol (15 mL, 3:1, v/v) in a round-bottomed flask and dried in a rotary evaporator (Rotavapor R-200, BUCHI Labortechnik AG, Switzerland) to form a thin lipid film. The final products were concentrated, lyophilized under vacuum (5 h) and stored (-20°C). The free liposomes were prepared the same way as liposomal quercetin was, except quercetin was not added. The diameter of liposomal quercetin was typically in the range 170±20 nm. In the following experiments, the listed dose of liposomal quercetin is based on the content of quercetin.
Effect of liposomal quercetin on acute hepatitis
Acute hepatitis was induced by injecting Con A (20 mg/kg; Sigma-Aldrich, USA) iv through the tail vein of female BALB/c mice. To investigate the role of quercetin in Con A induced-hepatitis, liposomal quercetin (2.5 mg/kg), free liposomes (3.75 mg/kg), or the same volume of saline were administered 30 min after Con A administration. Mice were sacrificed 4 or 24 h after Con A injection and blood samples and liver tissues were obtained. The levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured (Cobas Integra 400, F. Hoffman-La Roche Ltd., Switzerland).
Effects of liposomal quercetin on hepatic fibrosis
The hepatic fibrosis mouse model was induced as previously described (66. Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, et al. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol 1999; 11: 1491-1500, doi: 10.1093/intimm/11.9.1491.
https://doi.org/10.1093/intimm/11.9.1491...
,1616. Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 2003; 9: 347-351, doi: 10.1038/nm828.
https://doi.org/10.1038/nm828...
). Briefly, Con A (12.5 mg/kg) was injected iv into the tail vein of mice once a week for 6 consecutive weeks. Mice were treated with daily ip injections of liposomal quercetin (0.5 mg/kg), free liposomes (0.75 mg/kg), or the same volume of saline before being sacrificed 1 week after the final Con A injection.
Histopathology and immunohistochemistry
Liver tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Three-micrometer thick sections were stained with either hematoxylin and eosin or Sirius red for light microscopic evaluation. In sections that stained for collagen (Sirius red positive; magnification 100×), the areas were quantified by image analysis using the software Image-pro plus 5.0 (Media Cybernetic, USA). In the immunohistochemistry assay, an anti-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 monoclonal antibody (Santa Cruz Biotechnology, USA) was used as the primary antibody to detect the expression and distribution of NF-κB in liver tissue. An anti-α-SMA monoclonal antibody (Santa Cruz Biotechnology) was used as the primary antibody to analyze the activity of hepatic stellate cells.
RNA extraction and real-time RT-PCR
Frozen liver tissues were mechanically pulverized and total RNA was isolated with the Trizol reagent (Life Technologies, USA) to analyze NF-κB and transforming growth factor beta (TGF-β) mRNA expression. Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) of TGF-β and NF-κB was performed using a one-step kit (TaKaRa Biotechnology [Dilian] Co., Ltd., China). Samples were run in triplicate and quantitative analysis was performed according to the kit protocol. The sequences of the primers and TaqMan probes for mouse GAPDH, NF-κB p50 subunit, and TGF-β employed in this study are shown in Table 1. The reactions were performed at 42°C (15 min), 95°C (2 min), followed by 45 cycles at 95°C (30 s) and 50°C (31 s). Values were normalized to those of mouse GAPDH expression. Relative expression levels were analyzed using the comparative Ct method according to the TaqMan manual.
Statistical analyses
All data are reported as means±SD. Data were analyzed by one-way analysis of variance (ANOVA) and the least significant difference (LSD) test. Differences between means were considered significant at P<0.05. The experiments were performed at least in duplicate.
Results
Liposomal quercetin inhibited Con A-induced acute hepatitis
To evaluate acute hepatic injury after Con-A challenge, serum ALT and AST levels were measured. As shown in Figure 1A, iv administration of Con A resulted in a significant increase in serum ALT and AST 4 and 24 h after Con A administration. Liposomal quercetin treatment significantly decreased ALT and AST concentrations. Consistent with this observation, histological examination of liver sections showed that Con A challenge resulted in massive infiltration of inflammatory cells (4 h) and widespread areas of necrosis (24 h) in liver after Con A administration. Treatment with liposomal quercetin markedly attenuated these pathologic changes (Figure 1B). Moreover, free liposomes showed no effect on transaminase levels or pathologic changes. Our data indicate that liposomal quercetin reduced hepatocellular injury induced by Con A injection.
Liposomal quercetin (QU) inhibited acute hepatitis induced by Con A. Murine hepatitis was induced by iv injection of Con A. Liposomal QU, free liposomes (lipo), or saline were administered iv 30 min after Con A administration. Mice were sacrificed 4 or 24 h after Con A injection, and blood and liver tissue samples were collected. A, Levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) 4 and 24 h after Con A injection. Data are reported as means±SD for 6 mice per group. *P<0.01, compared to the Con A control (one-way ANOVA followed by the LSD test). B, Photomicrographs of representative H&E-stained liver sections. Black arrows indicate infiltration of inflammatory cells, and white arrows indicate necrotic areas.
Liposomal quercetin inhibited Con A-induced hepatic fibrosis
To assess the effect of liposomal quercetin on hepatic fibrosis, liver tissues were collected from mice 1 week after the sixth Con A injection and liver sections were stained with both hematoxylin and eosin and Sirius red for light microscopic analysis. Histological analysis showed collagen deposition and development of early-stage pseudolobuli after Con A challenge, whereas liposomal quercetin treatment markedly attenuated these changes (Figure 2A). To further quantitatively analyze collagen deposition, the Sirius red stained areas were analyzed using an Image-pro plus 5.0 software. Collagen deposition decreased from 7.36 to 1.75% in mice challenged with Con A and treated with liposomal quercetin (P<0.001, Figure 2B).
Liposomal quercetin (QU) inhibited Con A-induced hepatic fibrosis. Fibrosis was induced by iv injection of Con A once a week for 6 consecutive weeks in the tail vein of mice, which were treated by daily ip injections of liposomal QU, free liposomes (lipo), or saline. Mice were sacrificed 1 week after the last injection of Con A. A, Photomicrographs of representative H&E-stained liver sections. Black arrows indicate collagen deposition and development of early-stage pseudolobuli. B, Liver sections were stained with Sirius red to assess collagen deposition. To further quantitatively analyze collagen deposition, Sirius red-stained areas were analyzed using the Image-pro plus 5.0 software. Data are reported as means of the percentage of collagen positive areas and standard deviation. *P<0.01, compared to Con A control (one-way ANOVA followed by the LSD test). C, Liver sections were immunostained for α-SMA to assess HSCs activation.
The principal cells responsible for causing hepatic deposition of cross-linked fibrillar collagen are hepatic myofibroblasts (HmFs), which are believed to be largely derived from HSCs (1717. Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 2004; 1: 98-105, doi: 10.1038/ncpgasthep0055.
https://doi.org/10.1038/ncpgasthep0055...
). To further evaluate HSCs activation, immunohistochemical analysis for α-SMA was performed. As shown in Figure 2C, our results indicate that liposomal quercetin decreased the number of α-SMA positive cells, which is suggestive of inhibition of HSCs activation.
Quercetin inhibited the expression of NF-κB in the hepatic fibrosis model
In sections immunolabeled with the NF-κB p65 subunit, NF-κB was barely detected in the normal control group, whereas, it was significantly increased after Con A challenge. Moreover, NF-κB expression was observed mainly in liver HSCs/HmFs and immune cells. After mice received daily liposomal quercetin treatment, lower levels of NF-κB expression were detected (Figure 3A). In agreement with the immunohistochemical results, real-time RT-PCR analyses showed that NF-κB p50 subunit expression was significantly decreased in mice treated with liposomal quercetin compared with mice challenged with Con A (P=0.004; Figure 3B).
Quercetin inhibited the expression of NF-κB. A, Sections were immunostained for the NF-κB p65 subunit; the dark yellow or brown colors indicate NF-κB-positive cells. B, Results of real-time RT-PCR for the NF-κB p50 subunit. Data are reported as the means and standard deviation of the relative expression levels of NF-κB. QU: liposomal quercetin; lipo: free liposomes; normal: saline. *P<0.01, compared to Con A control (one-way ANOVA followed by the LSD test).
Quercetin inhibited the expression of TGF-β
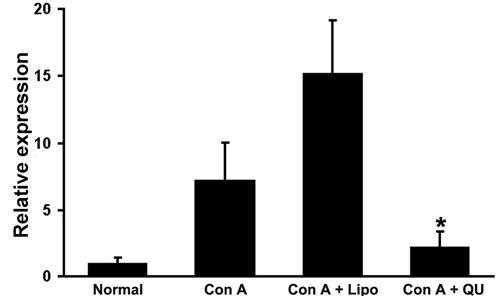
To estimate the relative expression of TGF-β, real-time RT-PCR was performed (Figure 4). After Con A challenge, TGF-β mRNA increased 7.2 times relative to normal control mice. Quercetin treatment reduced the increase in TGF-β expression to 2.2-fold (P=0.013, compared with mice challenged with Con A).
Quercetin inhibited expression of TGF-β. To estimate the relative expression level of TGF-β, real-time RT-PCR was performed. Data are reported as the means and standard deviation of the relative expression levels of TGF-β. *P<0.05, compared to Con A control (one-way ANOVA followed by the LSD test).
Discussion
Hepatic fibrosis is the result of wound-healing responses after liver injury caused by various etiologies, and immune mechanisms play an important role in this process. In the present study, we tested the effects of quercetin in hepatitis and hepatic fibrosis models induced by Con A. As shown in Figures 1 and 2, Con A challenge resulted in massive infiltration of inflammatory cells and widespread areas of necrosis. After liver injury, HSCs undergo a response known as “activation”, during which there is a transition from quiescent cells into proliferative, fibrogenic, and contractile myofibroblasts (1717. Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 2004; 1: 98-105, doi: 10.1038/ncpgasthep0055.
https://doi.org/10.1038/ncpgasthep0055...
). As we expected, treatment with liposomal quercetin markedly attenuated these pathologic changes.
Previous studies have suggested that NF-κB and TGF-β signals participate in liver damage and fibrosis induced by Con A (1818. Luedde T, Schwabe RF. NF-kappaB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8: 108-118, doi: 10.1038/nrgastro.2010.213.
https://doi.org/10.1038/nrgastro.2010.21...
19. Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007; 56: 284-292, doi: 10.1136/gut.2005.088690.
https://doi.org/10.1136/gut.2005.088690...
-2020. Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res 2012; 347: 245-256, doi: 10.1007/s00441-011-1246-y.
https://doi.org/10.1007/s00441-011-1246-...
), and therefore we tested the effects of quercetin on the NF-κB and TGF-β pathways. As shown in Figures 3 and 4, the expression of NF-κB and TGF-β was inhibited by lipsomal quercetin, suggesting that the inhibitory effect of quercetin on liver damage might be associated with its ability to modulate NF-κB and TGF-β production.
There is growing evidence that NF-κB acts as a key mediator of fibrosis in HSCs/HmFs, the main cells responsible for promoting hepatic deposition of cross-linked fibrillar collagen (1818. Luedde T, Schwabe RF. NF-kappaB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8: 108-118, doi: 10.1038/nrgastro.2010.213.
https://doi.org/10.1038/nrgastro.2010.21...
). HSCs/HmFs show activation of NF-κB during culture activation (2121. Elsharkawy AM, Wright MC, Hay RT, Arthur MJ, Hughes T, Bahr MJ, et al. Persistent activation of nuclear factor-kappaB in cultured rat hepatic stellate cells involves the induction of potentially novel Rel-like factors and prolonged changes in the expression of IkappaB family proteins. Hepatology 1999; 30: 761-769, doi: 10.1002/hep.510300327.
https://doi.org/10.1002/hep.510300327...
) and in human and mouse models of liver fibrosis (2222. Oakley F, Teoh V, Ching AS, Bataller R, Colmenero J, Jonsson JR, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 2009; 136: 2334-2344, doi: 10.1053/j.gastro.2009.02.081.
https://doi.org/10.1053/j.gastro.2009.02...
). NF-κB has been postulated to regulate three key aspects of HSCs/HmFs biology: activation, survival, and inflammatory response (2323. Oakley F, Meso M, Iredale JP, Green K, Marek CJ, Zhou X, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 2005; 128: 108-120, doi: 10.1053/j.gastro.2004.10.003.
https://doi.org/10.1053/j.gastro.2004.10...
). In the present study, a decreased NF-κB expression was observed in liver HSCs/HmFs from mice treated with liposomal quercetin, which is in agreement with previous studies reporting the effects of quercetin on NF-κB in other inflammatory models (99. Vicentini FT, He T, Shao Y, Fonseca MJ, Verri WA Jr, Fisher GJ, et al. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J Dermatol Sci 2011; 61: 162-168, doi: 10.1016/j.jdermsci.2011.01.002.
https://doi.org/10.1016/j.jdermsci.2011....
,2424. Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol 2005; 35: 584-592, doi: 10.1002/eji.200425778.
https://doi.org/10.1002/eji.200425778...
,2525. Yoon JS, Lee HJ, Choi SH, Chang EJ, Lee SY, Lee EJ. Quercetin inhibits IL-1beta-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy. PLoS One 2011; 6: e26261, doi: 10.1371/journal.pone.0026261.
https://doi.org/10.1371/journal.pone.002...
). Therefore, it is suggested that the inhibitory effect of quercetin on liver damage is associated with its ability to regulate NF-κB.
TGF-β plays an important role in liver fibrosis. Results of studies in which TGF-β is overexpressed in liver using adenovirus or in transgenic TGF-β mice have revealed that TGF-β contributes both HSCs activation and fibrogenesis (2626. Kanzler S, Lohse AW, Keil A, Henninger J, Dienes HP, Schirmacher P, et al. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol 1999; 276: G1059-G1068.
27. Ueberham E, Low R, Ueberham U, Schonig K, Bujard H, Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology 2003; 37: 1067-1078, doi: 10.1053/jhep.2003.50196.
https://doi.org/10.1053/jhep.2003.50196...
-2828. Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol 1999; 30: 77-87, doi: 10.1016/S0168-8278(99)80010-5.
https://doi.org/10.1016/S0168-8278(99)80...
). Furthermore, blocking of TGF-β signaling protected mice and rats against liver fibrosis in several experimental models (2929. Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 2006; 10: 76-99, doi: 10.1111/j.1582-4934.2006.tb00292.x.
https://doi.org/10.1111/j.1582-4934.2006...
,3030. Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, et al. Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 2013; 8: e54499, doi: 10.1371/journal.pone.0054499.
https://doi.org/10.1371/journal.pone.005...
). In the present study, quercetin inhibited TGF-β expression in liver with hepatic fibrosis, which is in agreement with other results showing that quercetin treatment of fibroblasts significantly inhibited collagen and TGF-β production (1010. Baowen Q, Yulin Z, Xin W, Wenjing X, Hao Z, Zhizhi C, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol 2010; 642: 134-139, doi: 10.1016/j.ejphar.2010.05.019.
https://doi.org/10.1016/j.ejphar.2010.05...
,3131. Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST, Longaker MT. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: implications for the treatment of excessive scars. J Trauma 2004; 57: 1032-1037, doi: 10.1097/01.TA.0000114087.46566.EB.
https://doi.org/10.1097/01.TA.0000114087...
,3232. Nakamura T, Matsushima M, Hayashi Y, Shibasaki M, Imaizumi K, Hashimoto N, et al. Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol 2011; 44: 614-620, doi: 10.1165/rcmb.2010-0338OC.
https://doi.org/10.1165/rcmb.2010-0338OC...
). Accordingly, the inhibitory effects of quercetin on hepatic fibrosis may be related to its ability to decrease TGF-β generation.
In summary, our results indicate that liposomal quercetin can effectively inhibit acute hepatitis and hepatic fibrosis induced by Con A. Furthermore, the present study showed that the inhibitory effect of quercetin was associated with its ability to modulate NF-κB and TGF-β production. These results suggest that liposomal quercetin may be a potent strategy in the treatment of patients with liver damage and liver fibrosis.
Acknowledgments
Research supported by the Scientific Research Foundation for Young Teachers (Sichuan University, #2010SCU11030).
References
-
1Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol 2005; 45: 605-628, doi: 10.1146/annurev.pharmtox.45.120403.095906.
» https://doi.org/10.1146/annurev.pharmtox.45.120403.095906 -
2Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008; 371: 838-851, doi: 10.1016/S0140-6736(08)60383-9.
» https://doi.org/10.1016/S0140-6736(08)60383-9 -
3Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010; 7: 425-436, doi: 10.1038/nrgastro.2010.97.
» https://doi.org/10.1038/nrgastro.2010.97 -
4Tanaka H, Leung PS, Kenny TP, Gershwin ME, Bowlus CL. Immunological orchestration of liver fibrosis. Clin Rev Allergy Immunol 2012; 43: 220-229, doi: 10.1007/s12016-012-8323-1.
» https://doi.org/10.1007/s12016-012-8323-1 -
5Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000; 275: 2247-2250, doi: 10.1074/jbc.275.4.2247.
» https://doi.org/10.1074/jbc.275.4.2247 -
6Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, et al. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol 1999; 11: 1491-1500, doi: 10.1093/intimm/11.9.1491.
» https://doi.org/10.1093/intimm/11.9.1491 -
7Valerio DA, Georgetti SR, Magro DA, Casagrande R, Cunha TM, Vicentini FT, et al. Quercetin reduces inflammatory pain: inhibition of oxidative stress and cytokine production. J Nat Prod 2009; 72: 1975-1979, doi: 10.1021/np900259y.
» https://doi.org/10.1021/np900259y -
8Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LF, Lemos-Senna E, et al. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res 2010; 61: 288-297, doi: 10.1016/j.phrs.2009.10.005.
» https://doi.org/10.1016/j.phrs.2009.10.005 -
9Vicentini FT, He T, Shao Y, Fonseca MJ, Verri WA Jr, Fisher GJ, et al. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J Dermatol Sci 2011; 61: 162-168, doi: 10.1016/j.jdermsci.2011.01.002.
» https://doi.org/10.1016/j.jdermsci.2011.01.002 -
10Baowen Q, Yulin Z, Xin W, Wenjing X, Hao Z, Zhizhi C, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol 2010; 642: 134-139, doi: 10.1016/j.ejphar.2010.05.019.
» https://doi.org/10.1016/j.ejphar.2010.05.019 -
11Pavanato A, Tunon MJ, Sanchez-Campos S, Marroni CA, Llesuy S, Gonzalez-Gallego J, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci 2003; 48: 824-829, doi: 10.1023/A:1022869716643.
» https://doi.org/10.1023/A:1022869716643 -
12de David C, Rodrigues G, Bona S, Meurer L, Gonzalez-Gallego J, Tunon MJ, et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol 2011; 39: 949-957, doi: 10.1177/0192623311418680.
» https://doi.org/10.1177/0192623311418680 -
13Kanter M. Protective effect of quercetin on liver damage induced by chronic toluene exposure in rats. Toxicol Ind Health 2012; 28: 483-491, doi: 10.1177/0748233711414613.
» https://doi.org/10.1177/0748233711414613 -
14Peres W, Tunon MJ, Collado PS, Herrmann S, Marroni N, Gonzalez-Gallego J. The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol 2000; 33: 742-750, doi: 10.1016/S0168-8278(00)80305-0.
» https://doi.org/10.1016/S0168-8278(00)80305-0 -
15Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res 2006; 12: 3193-3199, doi: 10.1158/1078-0432.CCR-05-2365.
» https://doi.org/10.1158/1078-0432.CCR-05-2365 -
16Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 2003; 9: 347-351, doi: 10.1038/nm828.
» https://doi.org/10.1038/nm828 -
17Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 2004; 1: 98-105, doi: 10.1038/ncpgasthep0055.
» https://doi.org/10.1038/ncpgasthep0055 -
18Luedde T, Schwabe RF. NF-kappaB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8: 108-118, doi: 10.1038/nrgastro.2010.213.
» https://doi.org/10.1038/nrgastro.2010.213 -
19Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007; 56: 284-292, doi: 10.1136/gut.2005.088690.
» https://doi.org/10.1136/gut.2005.088690 -
20Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res 2012; 347: 245-256, doi: 10.1007/s00441-011-1246-y.
» https://doi.org/10.1007/s00441-011-1246-y -
21Elsharkawy AM, Wright MC, Hay RT, Arthur MJ, Hughes T, Bahr MJ, et al. Persistent activation of nuclear factor-kappaB in cultured rat hepatic stellate cells involves the induction of potentially novel Rel-like factors and prolonged changes in the expression of IkappaB family proteins. Hepatology 1999; 30: 761-769, doi: 10.1002/hep.510300327.
» https://doi.org/10.1002/hep.510300327 -
22Oakley F, Teoh V, Ching AS, Bataller R, Colmenero J, Jonsson JR, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 2009; 136: 2334-2344, doi: 10.1053/j.gastro.2009.02.081.
» https://doi.org/10.1053/j.gastro.2009.02.081 -
23Oakley F, Meso M, Iredale JP, Green K, Marek CJ, Zhou X, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 2005; 128: 108-120, doi: 10.1053/j.gastro.2004.10.003.
» https://doi.org/10.1053/j.gastro.2004.10.003 -
24Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol 2005; 35: 584-592, doi: 10.1002/eji.200425778.
» https://doi.org/10.1002/eji.200425778 -
25Yoon JS, Lee HJ, Choi SH, Chang EJ, Lee SY, Lee EJ. Quercetin inhibits IL-1beta-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy. PLoS One 2011; 6: e26261, doi: 10.1371/journal.pone.0026261.
» https://doi.org/10.1371/journal.pone.0026261 -
26Kanzler S, Lohse AW, Keil A, Henninger J, Dienes HP, Schirmacher P, et al. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol 1999; 276: G1059-G1068.
-
27Ueberham E, Low R, Ueberham U, Schonig K, Bujard H, Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology 2003; 37: 1067-1078, doi: 10.1053/jhep.2003.50196.
» https://doi.org/10.1053/jhep.2003.50196 -
28Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo J Hepatol 1999; 30: 77-87, doi: 10.1016/S0168-8278(99)80010-5.
» https://doi.org/10.1016/S0168-8278(99)80010-5 -
29Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 2006; 10: 76-99, doi: 10.1111/j.1582-4934.2006.tb00292.x.
» https://doi.org/10.1111/j.1582-4934.2006.tb00292.x -
30Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, et al. Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 2013; 8: e54499, doi: 10.1371/journal.pone.0054499.
» https://doi.org/10.1371/journal.pone.0054499 -
31Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST, Longaker MT. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: implications for the treatment of excessive scars. J Trauma 2004; 57: 1032-1037, doi: 10.1097/01.TA.0000114087.46566.EB.
» https://doi.org/10.1097/01.TA.0000114087.46566.EB -
32Nakamura T, Matsushima M, Hayashi Y, Shibasaki M, Imaizumi K, Hashimoto N, et al. Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol 2011; 44: 614-620, doi: 10.1165/rcmb.2010-0338OC.
» https://doi.org/10.1165/rcmb.2010-0338OC
-
First published online June 24, 2014.
Publication Dates
-
Publication in this collection
24 June 2014 -
Date of issue
Aug 2014
History
-
Received
19 Nov 2013 -
Accepted
17 Mar 2014