Abstract
Each year, thousands of patients deal with ear, nose, and throat disorders that can be life threatening such as tracheal stenosis, or impact the psychosocial well-being, such as microtia. These often require surgical intervention using autologous or allogenic grafts. Tissue engineering represents an exciting alternative to substitute the use of human tissues, by fabricating living bioartificial constructs using three-dimensional scaffolds that incorporate or support human cells that can proliferate and mature. The complex geometries of ear, nose, and throat call for advanced fabrication techniques such as bioprinting, which leverages additive manufacturing to fabricate patient-specific scaffolds with a high design freedom and repeatability. Here, we will provide a comprehensive overview on the use of bioprinting technologies to address specific challenges in otolaryngology, with relevant examples from recent literature.
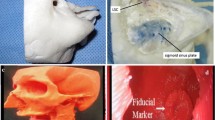
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Otolaryngology focuses on the treatment of ear, nose, and throat (ENT) disorders that affect thousands of patients worldwide every year.1 Those disorders could be life threatening, for example, tracheal stenosis, which affects important senses such as hearing and smell, and can impact the psychosocial well-being of the patient, for example, microtia and vocal folds (VFs) impairments.
Although the tissues/organs that are studied in otolaryngology are different, the gold standard treatment for their disorders is similar and based either on the use of cartilaginous auto- or allograft, or on the use of prosthetic and augmentation materials.2 However, both of these solutions present several drawbacks. Biological grafts suffer from donor-site morbidity, limited availability, and adverse immune response, whereas synthetic prostheses rarely lead to the regeneration of the targeted tissue.3 Hence, the interest in moving toward tissue engineering to fabricate bioequivalent substitutes ideally enable the host tissue to regenerate.4
The complex three-dimensional (3D) geometry of ENT organs poses a challenge to the fabrication of biomaterial scaffolds with interconnected pore networks that provide mechanical support for growing cells and developing tissue while matching the original tissue shape. Bioprinting technology then offers an exciting option for scaffold production, leveraging the possibility to manufacture highly reproducible, customizable, and even cell-laden structures.1,5,6,7
Bioprinting has in fact been defined as the application of additive manufacturing (AM) processes (i.e., joining materials layer by layer to make 3D objects starting from a 3D digital model) to fabricate scaffolds by the deposition and assembling of living and/or not living biomaterial(s) with an established organization.8 Bioprinting allows for a wide range of printing resolutions and an extensive library of materials availability, providing greater flexibility in shape creation than conventional methods and a high control over scaffold architecture and cell location. Moreover, bioprinting allows the fabrication of 3D scaffolds from volumetric information from patient medical images (e.g., computed tomography [CT]), and can arrange multiple biomaterials, cells, and biomolecules with high resolution, accuracy, and repeatability.9,10
Besides their complex 3D structures and microarchitectures, native tissues in otolaryngology also possess unique functions that are achieved through dynamic changes in tissue conformation. Therefore, the fabrication via 3D bioprinting of scaffolds is able to change, over time, their chemical and/or physical properties under a predefined stimulus (i.e., four-dimensional [4D] bioprinting),11 and could improve the potentiality of static scaffolds by mimicking the native tissue movements and simplifying their seeding.12,13
This article will provide an overview on bioprinting technologies for otolaryngology. After a brief description of the AM applied to otolaryngology, we will articulate the use of 3D and 4D bioprinting for the regeneration of ear, nose, larynx, vocal folds, and trachea, with relevant examples.
Bioprinting technologies
Bioprinting is a broad term that encompasses different AM technologies, which are described in detail elsewhere, including a comparison of their resolution and printing time.8,14 Here, we briefly describe the main AM technologies that have been applied to scaffold for otolaryngology, which are compared in Table I and schematically shown in Figure 1. The classification we implemented is in accordance with the standard ISO/ASTM 52900:2021.15
Commonly used additive manufacturing technologies. Image adapted with permission from Moroni et al.8 © 2018 Elsevier.
Binder (BJ) and material jetting (MJ) use an inkjet technology to deposit liquid droplets of a target material. In BJ (Figure 1a), the droplets are made of a liquid-bonding agent jetted onto a powder bed of the final material.16 Differently, in MJ (Figure 1b), a tool-head-based piezoelectric, thermal, acoustic, or valve-jet actuation deposits small droplets (pico- to nanoliters) of the material of interest on a target substrate.17
Conversely, vat photopolymerization (Figure 1c) uses a light source to initiate a polymerization reaction in specific locations of a layer of resin or monomer solution. After polymerization, a pattern inside the resin is solidified, which corresponds to the cross-sectional image of the final object. Vat photopolymerization techniques are generally classified as either stereolithography (SLA) or digital light processing (DLP). The former uses a focused laser beam to scan the surface of the cross-linkable resin, the latter uses a projector to cross-link a whole layer at a time.36
In material extrusion (ME), a pressure gradient is used to force the printable material through an orifice in a continuous strand deposited upon the printing plate or the previous layer, where it solidifies to create the final 3D object. The main ME technologies are fused deposition modeling (FDM, Figure 1d) and extrusion-based bioprinting (EBB, Figure 1e). In FDM, the material is heated at a temperature slightly below melting point, forced through the nozzle, and deposited on the printing bed layer by layer.8 Differently, in EBB processes, viscoelastic materials at low temperatures (room or physiological temperature), usually contained in a syringe, are extruded through a needle by pneumatic or mechanical methods.37
Recent advancements in bioprinting for otolaryngology
Ear
The human ear is a morphologically complex structure with critical functions, such as hearing and body balance, and cosmetic importance. It is composed of (1) the external ear that goes from the cartilaginous auricle to the ear canal; (2) the middle ear that comprises the tympanic membrane and the ossicles; and (3) the internal ear, where the bony labyrinth and the cochlea are located.1 The most common ear deformity is microtia, a congenital disorder affecting three to five infants out of 10,000; it is characterized by a spectrum of deformations of the external ear, ranging from milder variation to complete absence of the external ear.38 Surgical reconstruction is performed using cartilaginous autograft or alloplastic reconstruction39,40 to restore appearance, and ideally, function.41,42 Although these surgical methods have yielded great results, several important limitations remain, such as donor-site morbidity, foreign body reaction, and the non-restoration of biological function.43 In this scenario, TE, along with 3D bioprinting, offers an innovative and disruptive opportunity.44,45
For example, Jia et al.22 developed a biological auricular equivalent using a bioink made of (1) chondrocyte-laden methacrylate-modified acellular cartilage matrix (ACMMA), (2) methacrylate gelatin (gelMA), and (3) poly(ethylene oxide) (PEO), along with polycaprolactone (PCL). The ACMMA provided the bioink with a bioactive environment for cell activity, whereas the gelMA increased its printability; differently the PEO was used as sacrificial porogen, to allow the formation of micropores in the scaffolds, essential for nutrients’ exchange. PCL was introduced to provide mechanical support and higher morphological fidelity to the scaffold. With a multi-nozzle EBB, the authors were able to manufacture a human-like auricle, starting from the digital model of human auricula acquired by 3D laser scanning. In vivo tests in a nude mouse model showed the ability of the structure to maintain its shape, resisting skin tension. Moreover, histological assays, such as Safranin-O and Alcian Blue, revealed the formation of mature cartilage-like tissue with typical chondrocyte lacunae.
Exploiting a similar bioink, Xie et al.19 created a human-sized auricle construct via DLP (Figure 2a). The authors created a bioink made of gelMA, chondrocytes from the microtic auricle, and cartilage acellular matrix (CAM) microtissue, resulting in a highly biocompatible and easily DLP processable system. The 3D bioprinted structures were viable and flexible, with a strong texture and a fast rebound (Figure 2a[i, ii]). In vivo tests performed in nude mice revealed the formation of mature cartilage, with a more pronounced chondrogenic phenotype when compared to chondrocytes cultured in a mere gelMA hydrogel (Figure 2a[iii–iv]).
(a) Auricle bioprinting by Xie et al.: (i) Bioprinted solid auricular structure with GelMA and GelMA + CAM (cartilage acellular matrix) bioinks (scale bar full sample: 5 mm, scale bar close up: 1 mm) and their flexible properties. (ii) Viability of chondrocytes cultured in bioprinted constructs for 1, 10, and 20 days (***p < 0.001, ****p < 0.0001). (iii) Photos of the in vivo constructs at week 6 and week 12 (scale bar: 1 mm) in subcutaneous mouse model. (iv) mRNA expression analysis of chondrocytes in auricular constructs at week 12 (*p < 0.05, ****p < 0.0001). Reproduced with permission from Reference 19. © 2022 Wiley. (b) Ossicle bioprinting by Dairaghi et al. (i) Ossicle models printed on a solid support (from top to bottom, from left to right: malleus, incus, and steps). Scale bar 2 mm. (ii) Models printed with a mesenchymal stem cells-laden alginate-nanocellulose bioink. Scale bar 2 mm. Reproduced with permission from Reference 29. © 2022 MDPI.
In the context of microtia reconstruction via 3D bioprinting, the clinical trial of a patient-specific 3D bioprinted scaffold (referred to as AuriNovo) is currently recruiting test subjects.27 AuriNovo is a 3D bioprinted collagen-based scaffold encapsulating the patient's own auricular chondrocytes, to treat people born with severe microtia (Grades II–IV).
In addition to the auricle, the middle and internal ear are often damaged. For instance, tympanic membrane perforation is a serious health issue that could derive from acute and chronic otitis as well as traumatic injury.46 Such perforations are surgically treated with an autologous cartilage or fascia graft, but success rate is highly variable and depends on the skill of the surgeon, and how irregular the shape of the perforation.47 Thus, Kuo et al.28 combined the medical images obtained from endoscopy with EBB to bioprint-personalized acellular grafts to treat tympanic membrane perforations. The authors used a biomaterial ink made of gelMA and epidermal growth factor to support scaffold invasion in vivo. The healing effects of the fabricated acellular grafts were tested in vivo in a chinchilla model and micro-CT, and histology analysis revealed the repair of the perforate tympanic membrane with visible ongoing membrane remodeling in 28 days.
Focusing on a complete substitution of the tympanic membrane, Mota et al.23 combined electrospinning and FDM to fabricate a multiscale scaffold able to mimic the collagen fiber arrangement of the human tympanic membrane. The authors fabricated the framework of the construct combining specific radial and circular-patterned grooves via FDM exploiting a copolymer made of poly(ethylene oxide terephthalate) and poly(butylene terephthalate) (PEOT/PBT). This biomimetic pattern was designed to guide the deposition of cells along the directions created by the grooves. Then, a PEOT/PBT electrospun mesh was deposited by either (1) the “dual scale” approach, in which the electrospun mesh was placed on the top of the framework, or (2) the “triple scale” approach, in which the electrospun mesh was placed between the radial and circular patterns. Electrospinning fabrication parameters (such as needle-collector distance and applied voltage) were adjusted to control the thickness of the mesh and match the native tympanic membrane thickness (i.e., 30–100 µm). The authors also studied the cytocompatibility of the construct showing that cultured hMSCs remained viable, metabolically active, and effectively organized following the anisotropic character of the scaffold. Moreover, the dual- and triple-scale scaffolds resulted in a higher viability, cellularity, and protein content when compared with mere electrospun mesh, thus, proving the increased functionality of the multiscale approach.
Past the tympanic membrane, the middle ear ossicle (malleus, incus, and stapes), the smallest bones in the human body, can be damaged due to trauma or congenital deformations leading to partial or total deafness, as their function is to transmit sound from the tympanic membrane to the cochlea.48 The gold standard treatment for their replacement involves substitutes from cadavers or the use of metal prosthesis. However, the highly personalized, small, and complex anatomy of human ossicles limits the outcomes of these treatments.49,50 Thus, 3D bioprinting has opened new possibilities for ossicle reconstruction. For example, Dairaghi et al.29 exploited EBB to fabricate a realistic ossicle substitute with a paste made of calcium phosphate and hydroxyapatite (Figure 2b[i]). Due to the complex shape of the ossicle, the prints occurred into a sacrificial support bath (i.e., bioplotting technique51) made of a Carbopol hydrogel. The authors also tested the possibility to bioprint a mesenchymal stem cells (MSCs) laden alginate-nanocellulose bioink for the fabrication of living ossicles (Figure 2b[ii]).
Although highly advanced, none of the studies previously described aimed at directly restoring auditory sensing. In this context, in a pioneering work, Mannoor et al.30 exploited a multi-extruder bioprinter to combine structural (i.e., nonconductive silicone), biological (i.e., chondrocytes-laden hydrogel), and electronic (i.e., silver nanoparticle infused silicone) constituents. The authors realized an auricle-shaped structure made of chondrocytes-laden alginate with an integrated circular coil antenna connected to cochlea-shaped electrodes. The bioelectronic bionic ear was then cultured in vitro to enable cartilage tissue formation. To demonstrate the construct functionality, the authors performed a series of electronic characterizations that showed the system capability to receive electromagnetic signals over a wide frequency range, from Hz to GHz.
Nose
The nose’s primary physiological functions encompass the olfactory sense and the warming, humidifying, and cleaning of inhaled air.1 The nose structure is ensured by its main component, hyaline cartilage, which has low cellularity and a poor self-repair capacity.52 Nasal defects result from trauma, congenital defects, or oncological resection and could lead to both functional and cosmetic deficits. Autologous grafting and synthetic prostheses are currently the gold standards for treating nose defects, although their drawbacks point to the need for an innovative alternative, such as bioprinting.
Indeed, AM is ideal for manufacturing the complex geometry of the nose as AM provides great design freedom and capacity of scaffold customization to match individual patient features. For example, Nuseir et al.18 proposed a 5 h long digital workflow to directly design and fabricate a prosthetic nose using a Polyjet 3D printer (Table I). The workflow was applied to restore the esthetics of nasal loss in a 27-year-old woman and started with the digital reconstruction of the patient nose via CT-acquired images to then design the patient-specific nose prosthesis, which was 3D-printed with TangoPlus, as flexible and tear-resistant ink. External color pigmentation was applied to the structure to match the skin color of the patient. The 3D printed nose was retained in place using a bioadhesive and the patient reported satisfaction with the esthetic and comfort of the prosthesis.
This study showed the potential of AM to realize complex geometry and personalization, but was based on elastomers that do not lead to the regeneration of the tissue. Recently, we exploited EBB to fabricate instead human-size nose scaffolds for TE possessing interconnected micro- and macropores.31 Pectin, a green and biocompatible polysaccharide, was used as biomaterial ink, which we have shown to be biocompatible and to support a high viability of MSCs seeded on the scaffolds. Similarly, Lan et al.32 used a nasal chondrocytes-laden collagen I bioink to fabricate nose-shaped scaffolds via EBB. Due to the complex geometry of the structure, the print occurred in a sacrificial support bath of gelatin. The upregulated expression of several cartilage-related genes alongside the detection of collagen I and II in the constructs after 42 days of in vitro culture confirmed the synthesis of a cartilaginous matrix by the embedded chondrocytes.
Although these examples highlight innovative approaches for rhinoplasty, they only targeted the reconstruction of nasal cartilage for cosmetic purposes without reestablishing the nose sensory functionality. In this context, a pioneering study was carried out by Jodat et al.33 who integrated a bioprinted cartilage-like tissue with an electrochemical biosensing system to obtain both esthetic outcomes and functional olfactory sensation (Figure 3). The authors exploited pneumatic EBB to develop a multimaterial nasal construct alternating soft cell-laden layers for chondrogenic growth and stiff layers for mechanical robustness (Figure 3b), obtained by adjusting the respective concentrations of gelMA and poly(ethylene glycol) dimethacrylate. The hydrogels are highly biocompatible, as shown by Figure 3c). Moreover, the construct design included open nostril cavities to embed three biocompatible metal-based microelectrodes to form an olfactory microfabricated biosensor able to bind various chemical functional groups (Figure 3d).
Nose bioprinting by Jodat et al. (a) Schematic diagram of the fabrication approach and cell integration in the bioprinted construct. (b) Integration of the biosensing system with the 3D bioprinted construct: bioprinting phase, bioprinting outcome compared with the printing preview and digital model, and 3D bioprinted structure integrated with the biosensors in each nostril. (c) Confocal images of 3D soft gels immunostained with F-actin/DAPI on day 10 and day 30 of culture. The scale bars in the insets are 50 µm. (d) Bode plots using 0.1–1000 pg/mL of trinitrotoluene (TNT), sensing using ferrocyanide or culture media as electrolyte mediators. Reproduced with permission from Reference 33. © 2022 Wiley.
Larynx and vocal folds
The larynx is a sphincter in the upper airway that orchestrates swallowing, breathing, coughing, and voice.53 It is composed of several main cartilages interconnected by ligaments. Cancer and trauma are common reasons for the surgical removal of parts of or the full larynx, which is presently the only treatment for many complex larynx disorders.54 Hence, TE and bioprinting could offer great new opportunities to recreate the complexity of this organ.
A first attempt in this direction was made by Galliger et al.34 who developed a photocross-linkable bioink made of gelMA and decellularized cartilaginous extracellular matrix (dcECM). The addition of the dcECM increased the rheological features of gelMa in terms of viscosity, shear thinning behavior, and yield stress, thus allowing its bioprinting at room temperature without the need of external support. The authors exploited this innovative material to print a pediatric-size acellular larynx scaffold (21 mm in height, with a top flaring of 12 × 17 mm by an 8 × 12 mm diameter base). Micro-CT imaging confirmed the minimal variation of the scaffold from the digital model, with an average distance between the bioprinted structure and the digital model of 0.5 ± 0.4 mm.
Taking a step forward, Park et al.24 fabricated a cell-laden larynx by EBB, combining rabbit chondrocytes with a hydrogel based on gelMa and hyaluronic acid (Figure 4a[i]). First, the authors created a PCL framework for structure integrity with 200–500-µm-sized pores to allow cell invasion and nutrient transport. Then, the bioink was deposited in the space between the PCL framework, and subsequently cross-linked via UV irradiation. A fluidic system supplied basal medium to the building plate during the bioprinting process, thus lowering the temperature of the printed PCL and preventing bioink dehydration to improve cell survival (fabrication workflow in Figure 4a[ii]). The capacity of the larynx structure to replace a larynx defect was tested in vivo in a total laryngectomized rabbit model. Endoscopic examination showed that the inner surface of the scaffold was covered with newly formed connective tissue, and mucosal regeneration was observed 14 days after transplantation (Figure 4a[iii]). Moreover, the luminal diameter was well preserved without clear signs of stenosis. Histological analysis revealed that the construct maintained its structure postoperative until the end of the test (day 14) and the chondrocytes maintained their morphology and stained positive for collagen type II and Safranin-O (glycosaminoglycans) (Figure 4a[iv]).
(a) Larynx bioprinting by Park et al.: (i) 3D digital model of the larynx (gray = PCL framework, yellow = bioink) and bioprinted structure. (ii) 3D bioprinting process with the use of the fluidics supply system. (iii) Photographs of surgical procedures and endoscopic postoperative images of the transplantation area. (iv) Histology of the 3D bioprinted larynx after 1 and 2 weeks of in vivo transplantation. Reproduced with permission from Reference 24. © 2022 Wiley. (b) Vocal folds (VFs) bioprinting by Greenwood et al.: (i) Steps for fabricating the VF model via extrusion-based bioprinting. (ii) Front and lateral views of two 3D bioprinted VF models. (iii) Vibration tests: superior-view high-speed images at subglottal pressures of 0.89 kPa (top) and 2.60 kPa (bottom) and kymograms at 0.89 kPa (top) and 2.60 kPa (bottom). SEM, scanning electron microscopy; SLP, superficial lamina propria. Reproduced with permission from Reference 35. © 2021 Elsevier.
The VF, or vocal cords, are housed in the larynx and are the main driver of voice production.55 They stretch horizontally across the larynx and are constituted by (1) an outer squamous non-keratinizing stratified epithelium; (2) the lamina propria populated by fibroblasts that possess a density gradient that promotes the creation of mucosal waves responsible for good-quality phonation; and (3) the thyroarytenoid muscle that modifies the elongation and tension state of the VF. Vibration of VF result in sound production in a range between 100 and 3000 Hz,56 whereas VF dysfunctions from overuse, trauma, neurological disease, or congenital malformation lead to voice disorders. Usually, VF injuries are characterized by the fibrotic conversion of the ECM of the lamina propria, with an increase in stiffness, which leads to the disruption of the mucosal wave, and consequently, to phonation disorders.57 Despite being not vital, VF dysfunctions significantly affect the patient psychosocial activities, reducing its occupational performance and attendance58 and could be particularly impactful for children and their psychosocial development. Unfortuantely, at present, there are no surgical procedures available to regenerate the native stratified composition of the lamina propria.59
A first attempt to use bioprinting for VF engineering was made by Greenwood et al.35 who fabricated a functional multilayered VF model (Figure 4b). First, the authors created a mold with the shape of VF, then filled the mold with an epithelium-mimicking silicone, and finally, extruded by EBB different types of silicones within the epithelium-like one, to create a multilayered structure, possessing epithelium, lamina propria, and ligaments (Figure 4b[i–ii]). Vibration tests on the models showed a flow-induced vibration with several characteristics similar to human phonation (Figure 4b[iii]). High-speed images suggest the presence of an alternating convergent-divergent profile, a hallmark characteristic of human VF vibration.
Other than the stratified architecture, one of the main characteristics of the VF is their high resistance to the repeated and rapid oscillations required for phonation.60 To artificially mimic this peculiar property, Yang et al.20 developed a heterogeneous structure with complex and customized shape as well as a high fatigue resistance, able to withstand copious and sustained mechanical deformations. This structure comprised a holey polyacrylamide-poly(acrylic acid) hydrogel scaffold fabricated via SLA and strengthened by carboxyl-Fe3+. A softer hydrogel of polyacrylamide was then casted into the scaffold resulting in the topological entanglement of the polymeric networks. Thus, when the structure was stretched, the compliance of the matrix deconcentrated the stress in the scaffold, amplifying the fatigue, and under cyclical pressure achieved a fatigue threshold of 400 J/m2, withstanding approximately 50,000 deformation cycles.
Trachea
The trachea is a tube-shaped structure that forms a connection between the larynx and the lungs.61 It maintains an open conduit during respiration, cleaning and humidifying the inspired air before it reaches the lungs, provides elasticity during deglutition, and assists in speaking. It is a composite structure consisting, from the inner to the outer layer, of epithelium, basement membrane, connective tissue, smooth muscle, and cartilage, which is organized into 18 to 22 hyaline cartilage C-shaped rings.1,3 Trachea defects (e.g., congenital defects, stenosis, tumors, trauma) that require surgical treatment have been increasing in recent years62 and expanded (more than 50% of the trachea length) or complex defects require the replacement of a tracheal segment by grafts63 or synthetic implants.64,65 However, in the human body, there are no replacement tissue available to restore the authentic 3D complex structure of trachea, thus, limiting the use of autografts.66,67 On the other hand, the use of synthetic prosthesis, typically made of silicone, rarely conducts to tissue regeneration.68 Thus, there is much potential in the adoption of a TE approach to recreate the complex tracheal structure by bioprinting to customize the construct geometry with reproducible manufacturing.
For example, Kaye et al.25 fabricated via multi-extruder EBB a biodegradable multimaterial tracheal scaffold with a backbone consisting of a 270° PCL partial ring segment to provide mechanical stability, and multiple empty channels injected layer by layer with a chondrocytes-laden hydrogel. The constructs were tested in vivo in a rabbit model, showing adequate hyaline cartilage formation. Moving a step further, Park et al.26 3D-bioprinted a trachea scaffold comprising both an external cartilage phase and internal epithelial layer (Figure 5). The authors used a multi-extruder 3D bioprinter to fabricate on a rotating spindle a hollow, 20-mm-long, cylindrical scaffold comprising five different layers: (1) PCL framework; (2) epithelial cell-laden alginate; (3) PCL framework; (4) chondrocytes–laden hydrogel; and (5) PCL framework (Figure 5a–b). The innermost and outermost PCL layers possess a grid pattern to promote cell infiltration and neovascularization. Conversely, the middle PCL layer was solid to physically separate the epithelial and cartilaginous alginate layers. The viability of embedded cells as well as their correct positioning was evaluated in vitro via live/dead assays and cell tracker kits (Figure 5c). Then, the authors evaluated the in vivo therapeutic efficacy of this artificial trachea in a rabbit model that showed some degree of neonatal cartilage formation at 6 months after implantation (Figure 5d–e).
Trachea bioprinting by Park et al.: (a) Bioprinting system components and bioprinting process. (b) Photo of the bioprinted trachea. (c) Fluorescent microscopic image (green dye = epithelial cells—red dye = chondrocytes) revealing that the two hydrogel layers are completely separated. (d) Photo of the surgical procedures for the in vivo validation. (e) In vivo epithelium regeneration after 3, 6, and 12 months, showing the complete regeneration of the trachea epithelium after 6 months. Reproduced with permission from Reference 26. © 2019 Springer Nature.
Even more exciting is the work of Kim et al.21 who followed the 4D bioprinting approach to design a manufacture a self-folding trachea construct via DLP. To achieve the desired movement, the authors exploited the self-folding ability of a bilayer film, whose layer has differential swelling properties. The authors fabricated the higher hydrophilic layer with a chondrocytes-laden silk-gelMA hydrogel at low concentration, whereas the lower-hydrophilic layer was made of a high concentration of turbinate-derived cells in a silk-gelMA hydrogel. The structure was initially fabricated as flat layers that self-folded once hydrated, assuming a C-shaped geometry (degree of curvature ~80%) with turbinate-derived cells in the inside mimicking the mucus membrane of the trachea, and chondrocytes on the outside, mimicking the tracheal ring. Constructs were cultured in vitro for two weeks, after which both cell types were able to maintain their initial position and the chondrocytes maintained their phenotype that positively stained for Safranin-O. Finally, the authors tested the constructs in vivo in a rabbit model with a 210° defect. At eight weeks after implantation, the constructs were stably integrated with the host tissue, with regenerated epithelium already two weeks postoperatively. Moreover, the histological Safranin-O/Fast Green staining highlighted the formation of neo-cartilage in the outer layer of the trachea.
Conclusion
Tissue engineering opens new horizons in the treatment of airway disorders. When tissue engineering is combined with AM, it results in 3D bioprinting, which allows the fabrication of highly reproducible and accurate living scaffolds with a high control over architecture that can be based on the patient medical images. This approach has the potential to drastically improve the outcomes of surgical procedures in otolaryngology, where tissues are extremely complex in their shape and organization. In this article, we discussed encouraging results on the use of 3D bioprinting in otolaryngology, focusing on ear, nose, and throat. A clear hallmark of the success of the bioprinting approach in otolaryngology is given by the fact that one of the first clinical trials in bioprinting is in the treatment of microtia with AuriNovo, a 3D bioprinted collagen-based scaffold populated with the patient cells. Nevertheless, a number of improvements still need to be made in biomaterial performance, to achieve both biocompatibility and bioactivity, as well as mechanical performances that match those of native tissues. Moreover, the continuous innovation in fabrication technologies will lead to the development of improved methodology that will, for example, combine different approaches together, thus allowing the creation of graded, multimaterial, multiscale, and multicellular constructs that will more closely mimic the heterogeneity of native tissues. Similarly, advanced and innovative fabrication approaches, such as in situ bioprinting (i.e., the direct bioprinting of the construct on/into the patients), have the potential to revolutionize the “traditional bioprinting” (usually defined as in vitro bioprinting), especially for external anatomical districts as the ENT.
Similarly, the advancement of 4D bioprinting could also well represent a breakthrough in the TE for otolaryngology, by the fabrication of active structures able to mimic ear, nose, and throat tissue dynamics, in their development, physiology, and pathology state. Finally, an essential element that needs to be addressed to introduce bioprinting in the clinical practice is the establishment of an automatic tight control over the bioprinting process, so that the final bioprinted constructs possess a high quality and, as a consequence, could be compliant with EU regulations, FDA requirements, and relevant harmonized standards.
References
R. Di Gesù, A.P. Acharya, I. Jacobs, R. Gottardi, Connect. Tissue Res. 61(2), 117 (2020). https://doi.org/10.1080/03008207.2019.1663837
W.L. Niermeyer, C. Rodman, M.M. Li, T. Chiang, Laryngoscope Investig. Otolaryngol. 5(4), 630 (2020). https://doi.org/10.1002/lio2.416
T. Agarwal, I. Chiesa, D. Presutti, V. Irawan, K.Y. Vajanthri, M. Costantini, Y. Nakagawa, S.-A. Tan, P. Makvandi, E.N. Zare, E. Sharifi, C. De Maria, T. Ikoma, T.K. Maiti, Mater. Sci. Eng. C 123, 112005 (2021). https://doi.org/10.1016/j.msec.2021.112005
R. Langer, Pharm. Res. 14, 840 (1997). https://doi.org/10.1023/A:1012131329148
F. Zoccali, A. Colizza, F. Cialente, A. Di Stadio, I. La Mantia, C. Hanna, A. Minni, M. Ralli, A. Greco, M. de Vincentiis, Healthcare 11(1), 108 (2023). https://doi.org/10.3390/healthcare11010108
D. Tiwari, R.K. Vobilisetty, B. Heer, Indian J. Otolaryngol. Head Neck Surg. 74, 123 (2022). https://doi.org/10.1007/s12070-021-02634-5
N. Zhong, X. Zhao, Eur. Arch. Otorhinolaryngol. 274, 4079 (2017). https://doi.org/10.1007/s00405-017-4743-0
L. Moroni, T. Boland, J.A. Burdick, C. De Maria, B. Derby, G. Forgacs, J. Groll, Q. Li, J. Malda, V.A. Mironov, C. Mota, M. Nakamura, W. Shu, S. Takeuchi, T.B.F. Woodfield, T. Xu, J.J. Yoo, Trends Biotechnol. 36(4), 384 (2018). https://doi.org/10.1016/j.tibtech.2017.10.015
J. Groll, J.A. Burdick, D.-W. Cho, B. Derby, M. Gelinsky, S.C. Heilshorn, T. Jüngst, J. Malda, V.A. Mironov, K. Nakayama, A. Ovsianikov, W. Sun. S. Takeuchi, J.J. Yoo, T.B.F. Woodfield, Biofabrication 11(1), 013001 (2019). https://doi.org/10.1088/1758-5090/aaec52
I. Chiesa, C. Ligorio, A.F. Bonatti, A. De Acutis, A.M. Smith, A. Saiani, G. Vozzi, C. De Maria, Front. Med. Technol. 2, 571626 (2020). https://doi.org/10.3389/fmedt.2020.571626
T. Agarwal, S.Y. Hann, I. Chiesa, H. Cui, N. Celikkin, S. Micalizzi, A. Barbetta, M. Costantini, T. Esworthy, L.G. Zhang, C. De Maria, T.K. Maiti, J. Mater. Chem. B 9(37), 7608 (2021). https://doi.org/10.1039/d1tb01335a
C. Cimmino, L. Rossano, P.A. Netti, M. Ventre, Front. Bioeng. Biotechnol. 6, 190 (2018). https://doi.org/10.3389/fbioe.2018.00190
K. Uto, J.H. Tsui, C.A. DeForest, D.H. Kim, Prog. Polym. Sci. 65, 53 (2017). https://doi.org/10.1016/j.progpolymsci.2016.09.004
A.F. Bonatti, I. Chiesa, S. Micalizzi, G. Vozzi, C. De Maria, Minerva Orthop. 72(4), 376 (2021). https://doi.org/10.23736/S2784-8469.20.04032-1
International Organization for Standardization (ISO)/ASTM, Additive Manufacturing—General Principles—Fundamentals and Vocabulary (ISO/ASTM 52900, 2021)
M. Ziaee, N.B. Crane, Addit. Manuf. 28, 781 (2019). https://doi.org/10.1016/j.addma.2019.05.031
T. Boland, T. Xu, B. Damon, X. Cui, Biotechnol. J. 1(9), 910 (2006). https://doi.org/10.1002/biot.200600081
A. Nuseir, M.M. Hatamleh, A. Alnazzawi, M. Al-Rabab’ah, B. Kamel, E. Jaradat, J. Prosthodont. 28(1), 10 (2019). https://doi.org/10.1111/jopr.13001
X. Xie, S. Wu, S. Mou, N. Guo, Z. Wang, J. Sun, Adv. Healthc. Mater. 11(22), 2201877 (2022). https://doi.org/10.1002/adhm.202201877
H. Yang, M. Ji, M. Yang, M. Shi, Y. Pan, Y. Zhou, H.J. Qi, Z. Suo, J. Tang, Matter 4(6), 1935 (2021). https://doi.org/10.1016/j.matt.2021.03.011
S.H. Kim, Y.B. Seo, Y.K. Yeon, Y.J. Lee, H.S. Park, M.T. Sultan, J.M. Lee, J.S. Lee, O.J. Lee, H. Hong, H. Lee, O. Ajiteru, Y.J. Suh, S.-H. Song, K.-H. Lee, C.H. Park, Biomaterials 260, 120281 (2020). https://doi.org/10.1016/j.biomaterials.2020.120281
L. Jia, Y. Hua, J. Zeng, W. Liu, D. Wang, G. Zhou, X. Liu, H. Jiang, Bioact. Mater. 16, 66 (2022). https://doi.org/10.1016/j.bioactmat.2022.02.032
C. Mota, S. Danti, D. D’Alessandro, L. Trombi, C. Ricci, D. Puppi, D. Dinucci, M. Milazzo, C. Stefanini, F. Chiellini, L. Moroni, S. Berrettini, Biofabrication 7(2), 025005 (2015). https://doi.org/10.1088/17585090/7/2/025005
H.S. Park, J.S. Lee, C.-B. Kim, K.-H. Lee, I.-S. Hong, H. Jung, H. Lee, Y.J. Lee, O. Ajiteru, M.T. Sultan, O.J. Lee, S.H. Kim, C.H. Park, Bioeng. Transl. Med. 8(2), e10423 (2022). https://doi.org/10.1002/btm2.10423
R. Kaye, T. Goldstein, D.A. Grande, D. Zeltsman, L.P. Smith, Int. J. Pediatr. Otorhinolaryngol. 117, 175 (2019). https://doi.org/10.1016/j.ijporl.2018.11.010
J.-H. Park, J.-K. Yoon, J.B. Lee, Y.M. Shin, K.-W. Lee, S.-W. Bae, J. Lee, J. Yu, C.-R. Jung, Y.-N. Youn, H.-Y. Kim, D.-H. Kim, Sci. Rep. 9, 2103 (2019). https://doi.org/10.1038/s41598-019-38565-z
https://clinicaltrials.gov/ct2/show/NCT04399239?cond=microtia&draw=2&rank=4
C.Y. Kuo, E. Wilson, A. Fuson, N. Gandhi, R. Monfaredi, A. Jenkins, M. Romero, M. Santoro, J.P. Fisher, K. Cleary, B. Reilly, Tissue Eng. Part A 24(5–6), 527 (2018). https://doi.org/10.1089/ten.tea.2017.0246
J. Dairaghi, D. Rogozea, R. Cadle, J. Bustamante, L. Moldovan, H.I. Petrache, N.I. Moldovan, Appl. Sci. 12(21), 11015 (2022). https://doi.org/10.3390/app122111015
M.S. Mannoor, Z. Jiang, T. James, Y.L. Kong, K.A. Malatesta, W.O. Soboyejo, N. Verma, D.H. Gracias, M.C. McAlpine, Nano Lett. 13(6), 2634 (2013). https://doi.org/10.1021/nl4007744
A. Lapomarda, A. De Acutis, I. Chiesa, G.M. Fortunato, F. Montemurro, C. De Maria, M. Mattioli Belmonte, R. Gottardi, G. Vozzi, Biomacromolecules 21(2), 319 (2020). https://doi.org/10.1021/acs.biomac.9b01332
X. Lan, Y. Liang, E.J.N. Erkut, M. Kunze, A. Mulet-Sierra, T. Gong, M. Osswald, K. Ansari, H. Seikaly, Y. Boluk, A.B. Adesida, FASEB J. 35(3), e21191 (2021). https://doi.org/10.1096/fj.202002081R
Y.A. Jodat, K. Kiaee, D. Vela Jarquin, R.L. De la Garza Hernández, T. Wang, S. Joshi, Z. Rezaei, B.A.G. de Melo, D. Ge, M.S. Mannoor, S.R. Shin, Adv. Sci. 7(5), 1901878 (2020). https://doi.org/10.1002/advs.201901878
Z. Galliger, C.D. Vogt, H.R. Helms, A. Panoskaltsis-Mortari, Macromol. Mater. Eng. 307(10), 2200196 (2022). https://doi.org/10.1002/mame.202200196
T.E. Greenwood, S.L. Thomson, J. Biomech. 121, 110388 (2021). https://doi.org/10.1016/j.jbiomech.2021.110388
S. Corbel, O. Dufaud, T. Roques-Carmes, “Materials for Stereolithography,” in Stereolithography: Materials, Processes and Applications, ed. by P.J. Bártolo (Springer, Boston, 2011), pp. 141–159. https://doi.org/10.1007/978-0-38792904-0
A.F. Bonatti, I. Chiesa, G. Vozzi, C. De Maria, Bioprinting 24, e00172 (2021). https://doi.org/10.1016/j.bprint.2021.e00172
D.V. Luquetti, C.L. Heike, A.V. Hing, M.L. Cunningham, T.C. Cox, Am. J. Med. Genet. A 158A(1), 124 (2012). https://doi.org/10.1002/ajmg.a.34352
L. Soliman, M.R. Borrelli, N. Sobti, A.S. Woo, J. 3D Print. Med. 6(4), 195 (2022). https://doi.org/10.2217/3dp-2022-0018
S.D. Kozusko, P. Konofaos, R.D. Wallace, Ann. Plast. Surg. 85(1), 89 (2020). https://doi.org/10.1097/SAP.0000000000002213
A.L. Johns, R.E. Lucash, D.D. Im, S.L. Lewin, J. Plast. Reconstr. Aesthet. Surg. 68(4), 492 (2015). https://doi.org/10.1016/j.bjps.2014.12.019
D. Li, W. Chin, J. Wu, Q. Zhang, F. Xu, Z. Xu, R. Zhang, Aesthetic Plast. Surg. 34, 570 (2010). https://doi.org/10.1007/s00266-010-9502-1
E.M. Ronde, M. Esposito, Y. Lin, F.S. van Etten-Jamaludin, N.W. Bulstrode, C.C. Breugem, J. Plast. Reconstr. Aesthet. Surg. 74(12), 3235 (2021). https://doi.org/10.1016/j.bjps.2021.08.001
G. Zhou, H. Jiang, Z. Yin, Y. Liu, Q. Zhang, C. Zhang, B. Pan, J. Zhou, X. Zhou, H. Sun, D. Li, A. He, Z. Zhang, W. Zhang, W. Liu, Y. Cao, EBioMedicine 28, 287 (2018). https://doi.org/10.1016/j.ebiom.2018.01.011
S. Landau, A.A. Szklanny, M. Machour, B. Kaplan, Y. Shandalov, I. Redenski, M. Beckerman, O. Harari-Steinberg, J. Zavin, O. Karni-Katovitch, I. Goldfracht, I. Michael, S.D. Waldman, S.I. Duvdevani, S. Levenberg, Biofabrication 14(1), 015010 (2022). https://doi.org/10.1088/1758-5090/ac3b91
J. Kim, S.W. Kim, S.J. Choi, K.T. Lim, J.B. Lee, H. Seonwoo, P.-H. Choung, K. Park, C.-S. Cho, Y.-H. Choung, J.H. Chung, Tissue Eng. Part A 17(21–22), 2763 (2011). https://doi.org/10.1089/ten.tea.2010.0533
M.A. Ghanem, A. Monroy, F.S. Alizade, Y. Nicolau, R.D. Eavey, Laryngoscope 116(10), 1813 (2006). https://doi.org/10.1097/01.mlg.0000231742.11048.ed
D.V. Kumar, D.K. Chaitanya, V. Singh, D.S. Reddy, J. Anat. Soc. India 67(1), 12 (2018). https://doi.org/10.1016/j.jasi.2018.01.001
R. Saha, P. Srimani, A. Mazumdar, S. Mazumdar, J. Clin. Diagn. Res. 11(1), AC01 (2017). https://doi.org/10.7860/JCDR/2017/23906.9147
B.A. Neff, F.M. Rizer, A.G. Schuring, W.H. Lippy, Laryngoscope 113(9), 1525 (2003). https://doi.org/10.1097/00005537-200309000-00021
C. De Maria, I. Chiesa, D. Morselli, M.R. Ceccarini, S. Bittolo Bon, M. Degli Esposti, P. Fabbri, A. Morabito, T. Beccari, L. Valentini, Adv. Funct. Mater. 31(52), 2105665 (2021). https://doi.org/10.1002/adfm.202105665
C.M. Chiesa-Estomba, A. Aiastui, I. González-Fernández, R. Hernáez-Moya, C. Rodiño, A. Delgado, J.P. Garces, J. Paredes-Puente, J. Aldazabal, X. Altuna, A. Izeta, Tissue Eng. Regen. Med. 18, 343 (2021). https://doi.org/10.1007/s13770-021-00331-6
V. Lungova, S.L. Thibeault, Cell. Mol. Life Sci. 77, 3781 (2020). https://doi.org/10.1007/s00018-020-03506-x
S. Baiguera, A. Gonfiotti, M. Jaus, C.E. Comin, M. Paglierani, C. Del Gaudio, A. Bianco, D. Ribatti, P. Macchiarini, Biomaterials 32(19), 4433 (2011). https://doi.org/10.1016/j.biomaterials.2011.02.055
A.K. Miri, J. Voice 28(6), 657 (2014). https://doi.org/10.1016/j.jvoice.2014.03.001
I. Titze, T. Riede, T. Mau, PLoS Comput. Biol. 12(6), e1004907 (2016). https://doi.org/10.1371/journal.pcbi.1004907
J.M. Fishman, J. Long, M. Gugatschka, P. De Coppi, S. Hirano, S. Hertegard, S.L. Thibeault, M.A. Birchall, Laryngoscope 126(8), 1865 (2016). https://doi.org/10.1002/lary.25820
C. Ling, Q. Li, M.E. Brown, Y. Kishimoto, Y. Toya, E.E. Devine, K.-O. Choi, K. Nishimoto, I.G. Norman, T. Tsegyal, J.J. Jiang, W.J. Burlingham, S. Gunasekaran, L.M. Smith, B.L. Frey, N.V. Welham, Sci. Transl. Med. 7(314), 314ra187 (2015). https://doi.org/10.1126/scitranslmed.aab4014
J.K. Kutty, K. Webb, Tissue Eng. Part B Rev. 15(3), 249 (2009). https://doi.org/10.1089/ten.teb.2008.0588
J. Lohscheller, M. Doellinger, A.J. McWhorter, M. Kunduk, Ann. Otol. Rhinol. Laryngol. 117(7), 484 (2008). https://doi.org/10.1177/000348940811700703
P.W. Furlow, D.J. Mathisen, Ann. Cardiothorac. Surg. 7(2), 255 (2018). https://doi.org/10.21037/acs.2018.03.01
G. Damiano, V.D. Palumbo, S. Fazzotta, F. Curione, G. Lo Monte, V.M.B. Brucato, A.I. Lo Monte, Life (Basel) 11(7), 618 (2021). https://doi.org/10.3390/life11070618
M. Den Hondt, J.J. Vranckx, J. Mater. Sci. Mater. Med. 28, 24 (2017). https://doi.org/10.1007/s10856-016-5835-x
K.A. Kucera, A.E. Doss, S.S. Dunn, L.A. Clemson, J.B. Zwischenberger, ASAIO J. 53(4), 497 (2007). https://doi.org/10.1097/MAT.0b013e3180616b5d
X. Luo, Y. Liu, Z. Zhang, R. Tao, Y. Liu, A. He, Z. Yin, D. Li, W. Zhang, W. Liu, Y. Cao, Z. Guangdong, Biomaterials 34(13), 3336 (2013). https://doi.org/10.1016/j.biomaterials.2013.01.060
L.R. Bryant, B. Eiseman, J. Thorac. Cardiovasc. Surg. 48(5), 733 (1964). https://doi.org/10.1016/s0022-5223(19)33356-2
E.J. Propst, J.D. Prager, J. Meinzen-Derr, S.L. Clark, R.T. Cotton, M.J. Rutter, Arch. Otolaryngol. Head Neck Surg. 137(6), 583 (2011). https://doi.org/10.1001/archoto.2011.85
E.A. Agathos, P. Tomos, E. Lachanas, H. Gakiopoulou, A. Pantopoulou, D. Perrea, Asian Cardiovasc. Thorac. Ann. 18(6), 557 (2010). https://doi.org/10.1177/0218492310387448
Acknowledgments
We acknowledge funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health R21HL159521 and 1R56HL164536 (R.G.), Children’s Hospital of Philadelphia Research Institute (R.G.), and The Frontier Program in Airway Disorders of the Children’s Hospital of Philadelphia (R.G.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiesa, I., De Maria, C., Vozzi, G. et al. Three-dimensional and four-dimensional printing in otolaryngology. MRS Bulletin 48, 676–687 (2023). https://doi.org/10.1557/s43577-023-00544-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-023-00544-1