Abstract
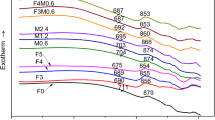
The crystallization of (9-X) K2O-1Li2O-12MgO-10B2O3-40SiO2-16Al2O3-12MgF2-X PbO/BaO/ZnO (X =0/5) composition (wt.%) were studied by means of dilatometry, DSC, XRD, SEM and microhardness analysis. Density of base K-Li-Mg-B-Si-Al-F glass (2.59 g.cm–3) is found to be increased on addition of the network modifier oxides PbO, BaO and ZnO content. Addition of Pb2+, Ba2+ and Zn2+ furthermore increased the glass transition temperature (Tg.). A characteristic exothermic hump is found to be appeared in DSC thermograph at the temperature range 800–950°C; and that is ascribed to the formation of crystalline phase fluorophlogopite mica, KMg3(AlSi3O10)F2. Opaque glass-ceramics were prepared from K-Li-Mg-B-Si-Al-F glasses (with and without containing PbO, BaO and ZnO content) by controlled heat-treatment at 1000°C. Interlocked type microstructure combined of flake like fluorophlogopite mica crystals is obtained in ZnO-containing K-Li-Mg-B-Si-Al-F glass-ceramic; and such microstructural pattern is ascribed to cause large thermal-expansion (>11.5×10-6/K, 50–800°C).Vickers Microhardness of base glass-ceramic (5.12 GPa) is increased when contains ZnO (5.26 GPa). ZnO-containing boroaluminosilicate glass-ceramic is, hence, considered with potential interest as they can exhibit the microcrack resistivity in high temperature recycling operation (like SOFC).
Similar content being viewed by others
References
S.N. Hoda, G.H. Beall, “Alkaline Earth Mica Glass-ceramics” In: J. H. Simmons, D. R. Uhlmann, G. H. Beall (Editors), Advances in Nucleation and Crystallization in Glasses, The American Ceramic Society, Westerville, pp. 287–300 (1982).
P. W. McMillan, G. Partridge, J. Mater. Sci. 7, 847 (1972).
W. Holand, G. H. Beall, Glass-Ceramic Technology, The American Ceramic Society, Westerville, Ohio, USA, 2002.
M. Garai, N. Sasmal, B. Karmakar, Ind. J. Mater. Sci., 638341, 1 (2015).
T. Hoche, S. Habelitz, I.I. Khodos, J. Cryst. Growth, 192, 185 (1998).
M. Garai, N. Sasmal, A. R. Molla, B. Karmakar, Solid state sci. 44, 10 (2015).
M. Kerstan, M. Muller, C. Russel, Mater. Res. Bull. 46, 2456 (2011).
Y.P. Tarlakov, I.F. Eskova, A.M. Shevyakov, Issled. Strukt. Sostyaniya Neorg. Veshchestu, 1, 7, (1974).
E.M.A. Hamzawy, H. Darwish, Mater. Chem. Phys. 71, 70 (2001).
C. B. da Silveira, S. D. de Campos, S. C. de Castro, Y. Kawano, Mater. Res. Bull. 34, 1661 (1999).
T. Yazawa, H. Tanaka, K. Eguchi, S. Yokoyama, J. Mater. Sci. 29, 3433 (1994).
J. E. Shelby, J. Applied Phys., 49, 5885 (1978).
S. Ghosh, P. Kundu, A. D. Sharma, R. N. Basu, H. S. Maiti, J. Eu. Ceram. Soc. 28, 69 (2008).
S. M. Salman, S. N. Salama, H. A. Abo-Mosallam, Ceramics International, 43, 9424 (2017).
M. Garai, N. Sasmal, A. R. Molla, S. P. Singh, A. Tarafder and B. Karmakar, J. Mater. Sci. 49, 1612 (2014).
E. Ercenk, S. Yilmaz, J. Ceram. Proc. Res. 16, 169 (2015).
M. Garai, B. Karmakar, J. Alloys Compd. 678, 360 (2016).
S. A. M. Abdel-Hameed, N. Ismail, H. F. Youssef, H. E. H. Sadek, M. A. Marzouk, International Journal of Hydrogen Energy, 42, 6829 (2017).
M. Garai, N. Sasmal, A. R. Molla, A. Tarafder, B. Karmakar, J. Mater. Sci.Tech.31, 110 (2015).
S. Gali, K. Ravikumar, B. V. S. Murthy, B. Basu, Dental Materials, 34, 36 (2018).
J. Henry, R. G. Hill, J. Non-crystalline Solids. 319, 13 (2003).
S. Roy, B. Basu, J. Mater. Sci.: Mater. Med. 21, 109 (2010).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garai, M., Maurya, A.K. & Roy, S. Zn2+- Controlled Crystallization and Microstructure in K-Li-Mg-B-Si-Al-F Glass. MRS Advances 3, 3525–3533 (2018). https://doi.org/10.1557/adv.2018.526
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2018.526