The term acute heart failure (AHF) describes the rapid onset or worsening of symptoms and/or signs of heart failure (HF) arising either de novo or, more frequently, as a consequence of acute decompensation of chronic HF. A wide range of possible causes or precipitants may initiate an episode of AHF; to that extent, AHF is an umbrella term for an extensive pathophysiological syndrome that may require widely differing clinical responses. In any event, AHF is a life-threatening situation that requires an urgent medical response, usually including speedy hospital admission.
AHF-related mortality rates are very high. In the European Society of Cardiology (ESC) Heart Failure Long-Term registry, which enrolled 12,440 patients from 21 European and/or Mediterranean countries, the 1-year mortality rate for AHF was almost fourfold that for chronic HF (23.6% versus 6.4%).1 The corresponding rates for the combined outcome of mortality or HF hospitalisation were 36% for AHF and 14.5% for chronic HF.1
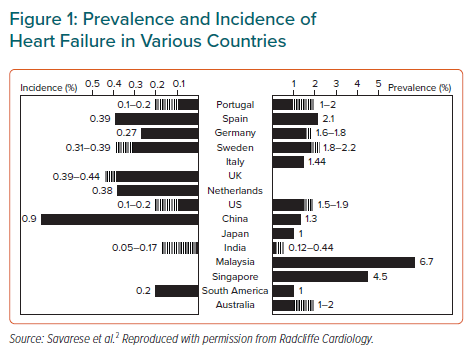
The burden of illness associated with AHF is encountered globally.2 As an exemplar of countries that have achieved notable economic progress in recent decades, China has recorded an increasing burden of coronary artery disease, hypertension, diabetes and obesity.3–5 This, combined with population aging, has created the conditions for many of the diseases of affluence to flourish. HF now affects >4 million individuals in China, and approximately 500,000 new cases are diagnosed every year.3 The prevalence and incidence rates of HF in China are comparable to those recorded in many other countries (Figure 1).2 However, it should be noted that many of these estimates are based on administrative data: echocardiography-based research in various countries returns higher percentages, as, for example, in the work of Guo et al. concerning northern China.6 Given these working estimates of prevalence and incidence, the implied morbidity and mortality rates for a population of >1 billion people is considerable, even though 1-year mortality in Chinese AHF patients was the lowest recorded in the Inter-CHF registry of low- and middle-income countries (7% versus an average of 16.5%).6
In an episode of AHF, when a patient decompensates despite optimal oral medications, IV cardioactive and vasoactive drugs may be required to stabilise the patient’s haemodynamics and augment peripheral perfusion. This is also necessary to address the involvement of systemic organs, such as the lungs, kidney and liver, which is typical of HF.7 However, the available repertoire of such drugs is relatively narrow, and evidence for their sustained benefits is limited, inconclusive and often unpersuasive, especially with regard to longer-term effects on morbidity and survival.8 There have been strikingly few successful introductions in this field of therapeutics in recent times; some of us (DF, FF, PP) have contributed to a recent survey of the field that includes an overview of some of the reasons for this lack of success.8
Levosimendan is a notable exception to the frustrations associated with drug development in this field and has recently marked two decades in mainstream clinical use in many countries of Europe and elsewhere for the management of AHF.9 A consideration of its properties and clinical impact is therefore relevant as part of a wider discussion about the development of HF care in China.
The Inodilator Levosimendan
IV levosimendan (SIMDAX®) emerged from a research and development program by Orion Pharma (Espoo, Finland) that focused on expanding treatment options for patients with AHF or acutely decompensated HF (ADHF).
IV levosimendan is notable for the fact that its inotropic effects are exerted through calcium sensitisation, not calcium mobilisation.10 This novel mode of action, which clearly differentiates it from adrenergic inotropic agents, made levosimendan a first-in-class agent at the time of its introduction, and it remains the only drug of its kind widely approved for clinical use.11–14 A recently published 20-year perspective on levosimendan is instructive.9
In addition to its calcium-sensitising effect, levosimendan mediates the opening of ATP-dependent potassium (KATP) channels in vascular smooth muscle cells.15 This causes systemic vasodilatation at usual therapeutic doses, and the drug should therefore be regarded as an inodilator rather than an inotrope. Levosimendan also opens KATP channels in mitochondria, exerting potentially cardioprotective effects.16–19
Several reports have shown that the vasodilator effect of levosimendan, and possibly its antioxidant and anti-apoptotic effects, are mediated by a mechanism involving both the opening of mitochondrial KATP channels and the modulation of nitric oxide release by different nitric oxide synthase isoforms.20–22
Importantly, the combined effects of levosimendan on cardiomyocytes, the coronary circulation and cardiac mitochondria have a favourable effect on the overall energy balance of cardiac function, which has not been shown for any other inodilator.23
Various other ‘pleiotropic’ actions of levosimendan may theoretically be relevant in certain scenarios, but further studies are needed before firm conclusions can be reached about their broader clinical significance.24–26 These properties of levosimendan will not be discussed further in this review, but Chinese medical scientists at Zhejiang University have produced a commentary that will be informative for readers interested in those topics.27
Clinical Experience with Levosimendan
Levosimendan has been extensively evaluated for the management of AHF in Europe in placebo- and active-controlled studies, and currently has marketing authorisation as SIMDAX in >60 countries.28–31 Detailed clinical evaluations have also been conducted recently in China, and instructive comparisons may be made between trials performed in China and those conducted elsewhere.
A summary of key findings of the first generation of major clinical trials of levosimendan is a useful starting point for such a comparison.
First, levosimendan resulted in dose-dependent increases in cardiac output and stroke volume, accompanied by reductions in pulmonary capillary wedge pressure (PCWP), mean blood pressure (BP), mean pulmonary artery pressure, mean right atrial pressure and total peripheral resistance.32 These effects persisted after termination of drug administration due to a long-acting metabolite designated OR-1896.
Second, rapid and sustained reductions in levels of natriuretic peptides were seen in response to an infusion of levosimendan.32 (This effect has been corroborated in a meta-analysis of studies in AHF undertaken by Chinese investigators at Chongqing Medical University.33) Third, there were consistent benefits in symptom control with levosimendan.28,30 However, there were mixed findings for hospitalisation and mortality: positive numerical trends were observed, but did not attain statistical significance.29,31
Fourth, as summarised by Yilmaz et al., numerous studies have indicated an improvement in renal function with levosimendan in AHF.25 However, this finding must be interpreted after consideration has been given to the variation in study designs and the lack of any robust effect in the REVIVE program.30 Technical and mechanistic studies suggest that levosimendan is differentiated from agents such as dobutamine by the fact that it exerts a preglomerular vasodilator action via its effect on KATP channels in arteriolar vascular smooth muscle cells.34 A renoprotective effect of pharmacological preconditioning has also been proposed.35
Finally, the safety of levosimendan in predominantly non-Chinese patients with AHF has been the subject of a meta-analysis (n=5,349) conducted by Gong et al. from the First Affiliated Hospital of Jinan University.36 In that study, Gong et al. concluded that there were increased risks of recurrence of extrasystoles (RR 1.88; 95% CI [1.26–2.81]; p=0.002); headache or migraine (RR 1.94; 95% CI [1.54–2.43]; p<0.00001) and hypotension (RR 1.33; 95% CI [1.15–1.53]; p=0.0001) with levosimendan compared with combined control therapy comprising placebo or dobutamine. Levosimendan also lowered systolic BP (SBP) to a significantly greater extent than placebo or dobutamine (p≤0.02).
Levosimendan Clinical Research in China
Acute Heart Failure Studies
The first multicentre randomised active-controlled parallel-group study of levosimendan in China for the management of AHF was reported by Wang et al.37 In that study, 225 patients with decompensated HF refractory to conventional therapy were randomised to treatment with either levosimendan (12 µg/kg over 10 minutes, followed by continuous infusion of 0.1 µg/kg/min for 1 hour and then 0.2 µg/kg/min for 23 hours; n=119) or dobutamine (2 µg/kg/min for 1 hour and then 4 µg/kg/min for 23 hours; n=106) for 24 hours. Haemodynamic responses at 24 hours were evaluated using echocardiography in both groups and a Swan–Ganz catheter in the levosimendan group.37
The end-of-treatment haemodynamic findings in that study favoured levosimendan, with numerically more frequent improvement in left ventricular ejection fraction (LVEF) in the levosimendan than dobutamine group (6.4% versus 4.6%; p>0.05) and significantly greater stroke volume enhancement with levosimendan (+11.1 versus +2.8 ml; p<0.05).37 The ‘clinical effectiveness rate’ was also significantly higher in the levosimendan than dobutamine group (32% versus 18%; p<0.01) and there were significantly (p<0.05) fewer adverse events in the levosimendan group, including hypokalaemia, hypotension and ventricular premature beats. Overall, levosimendan was well tolerated and both haemodynamically and clinically superior to dobutamine.
In a study by Zhang et al., 114 patients with ADHF were randomised to receive levosimendan for 24 hours and another 114 were randomised to dobutamine.38 The ejection fraction increased by ~3% in both groups, but PCWP decreased significantly more in the levosimendan than dobutamine group (−8.90 ± 7.14 mmHg versus −5.64 ± 6.83 mmHg; mean ± SD; p=0.04), as did the plasma N-terminal pro B-type natriuretic peptide (NT-proBNP) concentration (−22.36 ± 38.98% versus −8.56 ± 42.42%; mean ± SD; p<0.01). The improvement in dyspnoea at the conclusion of drug infusion was significantly more pronounced in the levosimendan group. The incidence of adverse reactions was similar in the two groups.
The same authors confirmed these findings in another randomised control study, also reporting a significantly more pronounced increase of the cardiac index from baseline value in the levosimendan group when compared to the dobutamine group.39
Acute Heart Failure Associated With MI
Two studies published simultaneously in 2014 have been conducted in this field of cardiology in China.40,41
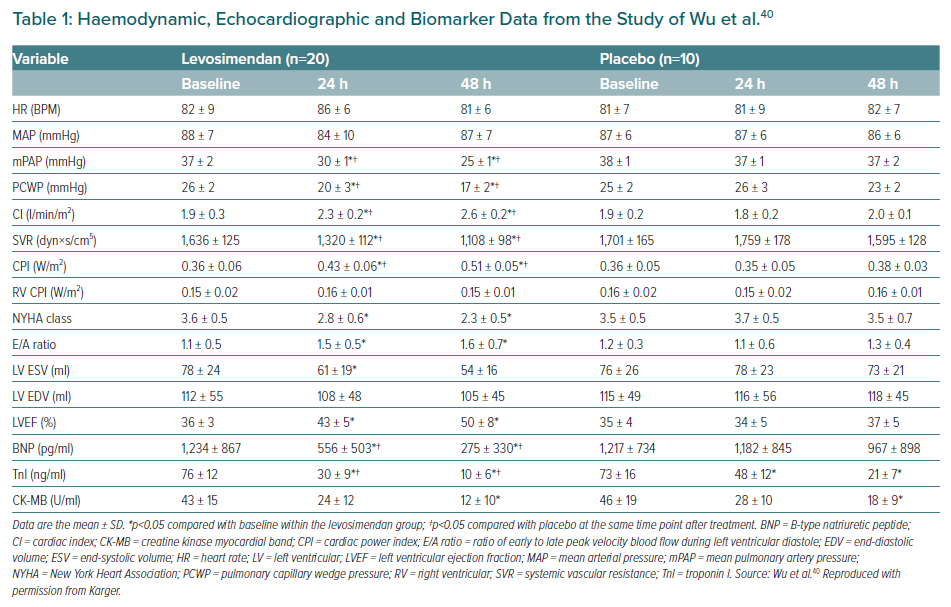
In a pilot study by Wu et al., 30 consecutive patients with an acute MI (AMI) who exhibited signs of myocardial stunning despite undergoing an emergency percutaneous coronary intervention within 12 hours after the infarction were enrolled in an open-label randomised placebo-controlled trial.40 Twenty patients were randomised to receive levosimendan (0.1 µg/kg/min for 24 hours), and 10 were assigned to matching placebo. All patients were in New York Heart Association (NYHA) class III or IV at baseline, all were taking aspirin or other antiplatelet drugs, angiotensin-converting enzyme inhibitors and diuretics and most (70–85% depending on treatment group assignment) were also taking β-blockers and spironolactone.40 Patients in the two groups were also well matched regarding the location of index infarction and pre-intervention coronary blood flow status. Comparison across multiple indices of haemodynamic performance and biomarkers identified broad-ranging statistically significant effects of levosimendan (Table 1). In addition, echocardiographic analysis established a lower percentage of stunned or infarcted myocardial segments in the levosimendan group (p<0.05 and p<0.01, respectively).
In a study reported in the same issue of Cardiology, Jia et al. described the recruitment of 160 patients with HF complicated by AMI to a randomised single-centre single-blind study.41 In contrast with the study of Wu et al., this study was designed to address the significant proportion of patients with AMI who had not undergone cardiac revascularisation procedures, thus providing similarities to the enrolled population of the RUSSLAN study.29 Performed at 21 centres in Latvia and Russia, RUSSLAN was the principal regulatory study to examine the impact of SIMDAX-brand levosimendan in patients with AHF/AMI.
For their study, Jia et al. selected patients who had AMI diagnosed according to established criteria during the previous 14 days, LVEF <40%, Killip class II–IV and either or both of: dyspnoea at rest and/or need for mechanical ventilation for ADHF; and non-hypovolaemic oliguria.41 Eligible patients were randomised in a 1:1 ratio to receive levosimendan (0.1 µg/kg/min for 24 hours) or placebo, and treatment effects were assessed via a composite primary endpoint that included death, myocardial ischaemia or worsening HF at both 14 days and 6 months.
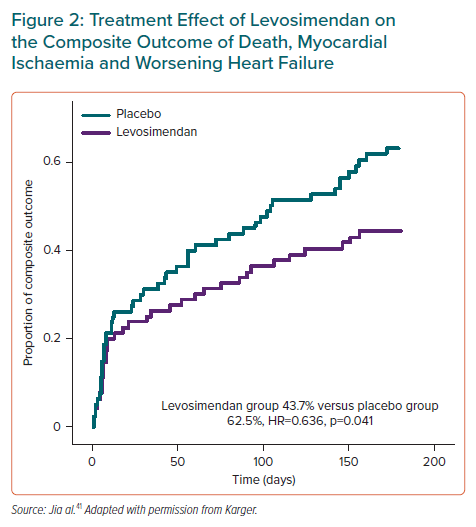
Although the percentage of patients who died or experienced worsening HF at 14 days was lower and the percentage of patients with myocardial ischaemia was higher in the levosimendan group, the differences did not reach statistical significance.41 Overall, the percentage of patients meeting the primary composite endpoint criteria at 14 days was lower, albeit not significantly, with levosimendan (21.2% versus 26.2%).41
At 6 months, the non-significant trends for death and worsening HF were supplemented by a significant trend for a lower incidence of MI in the levosimendan group between 14 days and 6 months (3.8% versus 13.8%; HR 0.261; p=0.036).41 As a result, there was a significant treatment effect of levosimendan on the composite primary endpoint (43.7% versus 62.5%; HR 0.636; 95% CI [0.413–0.981]; p=0.041; Figure 2).41 These findings are strikingly similar to those of the RUSSLAN trial.29
Stratification of outcomes by Killip class and revascularisation status indicated that favourable treatment effects of levosimendan were more likely to be recorded in patients in Killip class II (29.0% versus 52.6%; p=0.066) or in those who had undergone revascularisation (40.9% versus 67.4%; p=0.037).41
There was also a marked reduction in B-type natriuretic peptide (BNP) in response to levosimendan infusion and an enhancement of LVEF was apparent within 3 days of treatment (p=0.008 versus the control group).
Chronic or Refractory Heart Failure
The impact of a single 24-hour infusion of levosimendan on the clinical status of elderly patients with refractory HF was investigated in a two-stage study undertaken by researchers at Chongqing Medical University.42 In the first phase of this trial, 268 patients were assigned to maximal conventional HF therapy if they fulfilled the following criteria: age >70 years, NYHA Class III–IV, LVEF ≤40% and plasma NT-proBNP ≥1,000 pg/ml. Patients (n=42) who still met all these criteria after 2 weeks were then divided into two groups well matched with regard to demographic criteria, and were randomly assigned to a continuation of standard therapy or to standard therapy plus an infusion of levosimendan (12 µg/kg for 10 min, followed by continuous infusion of 0.1 µg/kg/min for 1 hour and then 0.2 µg/kg/min for 23 hours).42 It is not clear from the original report whether the comparator group received a matching placebo infusion.
At assessment 1 week later, all 21 of the levosimendan-treated patients were classified in NYHA Class I or II, compared with 1 of 21 patients in the control group (p<0.05).42 Comparisons of changes in LVEF and NT-proBNP were also robustly in favour of levosimendan.42 Levosimendan was reported to be well tolerated in that study, but the authors acknowledged that the small patient numbers and the short duration of follow-up limited their ability to draw firm conclusions.42
Practical Issues Concerning the Use of Levosimendan in the Chinese Clinical Setting
Cui et al. have recently commented on the need to develop an efficient HF management program in China, highlighting, among other things, the “importance of establishing the most cost-effective prevention and therapy strategies”.43 Defining the role and usage of levosimendan may be seen as one aspect of this requirement.
The Chinese Society of Cardiology is affiliated with the ESC and its 2018 guidelines closely reflect the ESC recommendations.44,45 The uses of levosimendan identified in both sets of guidelines include:
- short-term, IV use in patients with hypotension (systolic blood pressure [SBP] <90 mmHg) and/or signs or symptoms of hypoperfusion despite adequate filling status to increase cardiac output and BP, improve peripheral perfusion and maintain end-organ function (recommendation grade IIb C); and
- reversing the effect of β-blockade if β-blockade is thought to be contributing to hypotension with subsequent hypoperfusion (recommendation grade IIb C).
To these indications may be added the possibility of using intermittent infusions of levosimendan for long-term symptom relief or control in cases of AHF. This use is acknowledged by the Heart Failure Association of the ESC, which recognises the advantage of the prolonged haemodynamic effect conferred by the metabolite OR-1896.46
The use of levosimendan in Chinese patients for the management of AHF broadly conforms to international practice. For that indication, the drug is given as a continuous infusion of 0.05–0.2 μg/kg/min for 24 hours. Infusion may be preceded by a loading dose of 6–12 μg/kg over 10 min if an instant effect is sought, as long as baseline SBP is adequate.44,45 The use of an initial loading dose of levosimendan is not currently recommended, but was used in at least one of the Chinese trials described previously.37
Infusion is most often commenced initially at a dose of 0.1 µg/kg/min (or 0.05 µg/kg/min when SBP is marginal) and then up-titrated to 0.2 µg/kg/min after the first 2–3 hours, provided that satisfactory BP is maintained. The recommended duration of infusion in AHF is 24 hours. If a faster onset of action is required, treatment can be initiated at a dose of 0.2 μg/kg/min, which has an acceptable risk–benefit profile for infusions of up to 6 hours.47
Hypovolaemia and hyperkalaemia should be corrected before infusion, and volume and potassium status monitored during treatment. In complicated cases, a pulmonary artery (Swan–Ganz) catheter may be considered to monitor filling pressures and cardiac output.48 These requirements profile levosimendan as a drug most appropriately used by experienced physicians, probably in tertiary cardiology centres.
HF is part of an emerging cardiovascular health challenge in China. Initiatives such as the China-PEACE study (NCT02877914) and the HERO registry have identified several new themes that may be pertinent to the successful deployment of levosimendan.3,49–53
First, the use of several standard classes of drugs in HF patients in China is lower than that in international registries.53 In particular, the use of β-blockers currently appears to be notably low.53 The population of HF patients in China being treated with β-blockers can be expected to expand considerably in the coming years. If this is indeed the case, then the population of patients for whom levosimendan may be the preferred inotropic therapy can be expected to increase over time.
Second, a large proportion of HF cases in China appear to have good LVEF (≥50%), so-called HF with preserved EF (HFpEF).53 Levosimendan has not been extensively studied in this segment of the HF population, and a meta-analysis of outcomes in patients undergoing cardiac surgery identified no survival benefit in those with preserved LVEF (RR 1.06; 95% CI [0.72–1.56]; p=0.78) compared with studies of patients with moderate and low LVEF (RR 0.44; 95% CI [0.27–0.70]; p<0.001).54 However, mortality reduction may not be the only legitimate aim of short-term treatment, and there are indications of the potential of levosimendan to improve diastolic function from a small prospective randomised study of patients undergoing aortic valve surgery.55 Some commentators think the latter may be relevant to HFpEF.56 In addition, a modest haemodynamic response has recently been reported from the Phase 2 HELP trial (NCT03541603) and more insights may emerge from its open-label extension (NCT03624010). Pending further research, the use of levosimendan in patients with HFpEF should be restricted to people fulfilling current guideline criteria and in whom active haemodynamic monitoring is performed.
Finally, attention must be given to the widespread use of traditional Chinese medicines (TCMs) in the management of HF patients. These compounds have a prominent place in the Chinese health ecosystem, and it would be misplaced to dismiss them. However, few TCMs have been subjected to Western-style assessment and, although plans are in hand for a systematic review and network meta-analysis of HF-targeted TCMs, those plans are at an early stage.57 Given that levosimendan will, in most cases, be administered as a short-term treatment, the potential for meaningful adverse interactions with TCMs would appear to be limited. However, there is a need for care in monitoring and recording such interactions until potential hazards have either been identified or can be discounted. This requirement may gain further impetus if levosimendan becomes widely used in China as a recurring intermittent infusion to support people with episodic decompensations or with advanced HF.
Expert Assessment
We opened this short review with a list of the key characteristics of levosimendan in acute or decompensated HF which, with experience in China duly noted, may now usefully be revisited.
Haemodynamics
Levosimendan has consistently been shown to exert a range of favourable effects on several haemodynamic indices, including LVEF, cardiac index and PCWP, as well as on echocardiographic indicators of ventricular function. These effects were apparent in the series of pivotal regulatory studies conducted in Europe and have been recapitulated in the Chinese studies reviewed in this paper.
Natriuretic Peptides
Rapid, early and sustained reductions in BNP constitute a constant feature of the response to levosimendan in patients with acute or decompensated HF, and studies in Chinese patients confirm this effect, as discussed earlier. For example, a meta-analysis of randomised controlled trials in patients with advanced HF by Cui et al., from Chongqing Medical University, has confirmed this effect of levosimendan and associated it with improvement in LVEF compared with placebo, dobutamine, furosemide and prostaglandin E1 (standardised mean difference 0.74; 95% CI [0.22–1.25]; p=0.005).33
All in all, an effect on natriuretic peptide concentrations may be considered as one of the means for discriminating between levosimendan responders and non-responders (i.e. those in whom levosimendan has robust natriuretic peptide level decreasing effects, and those in whom this effect was not present, respectively).58 This reflects the general correlation existing between decreases in natriuretic peptide and treatment efficacy in HF, not only for levosimendan.59
Symptoms
Relief of HF-related symptoms such as dyspnoea is a robust finding in clinical studies of levosimendan in acute or decompensated HF: experience in the REVIVE trial may be considered as an exemplar of this fact, which has been broadly replicated in the Chinese studies we have summarised.
Renal Effects
Regarding AHF with accompanying renal dysfunction, Chinese data consistent with observations in non-Chinese populations are already available.34,60–62
A further contribution to understanding the renal effects of levosimendan may be found in the work of Chen et al., who conducted a network meta-analysis of 29 published trials to provide direct comparisons of levosimendan versus placebo or inotropes in patients undergoing cardiac surgery.63 They concluded that, compared with placebo, the use of levosimendan significantly decreases the risks of mortality (OR 0.74; 95% CI [0.56–0.97]) and acute renal injury (OR 0.61; 95% CI [0.45–0.82]), especially in patients with low systolic function.63 Comparisons with milrinone, dopamine, dobutamine and fenoldopam identified levosimendan as the best treatment based on p-values.
In an associated development, the authors of a recently reported retrospective cohort study conducted in Taiwan showed that an estimated glomerular filtration rate of <30 ml/min/1.73 m2 was not necessarily a contraindication to the use of levosimendan in patients with AHF and reduced EF, albeit no substantive effect was observed on 30- to 180-day mortality.64
Survival
Effects on mortality remain the subject of debate, and the lack of a significant improvement in large regulatory studies offsets some of the more optimistic findings from meta-analyses, as in the work of Gong et al. and Pollesello et al.36,65 The meta-analysis and trial sequential analysis of randomised trials of levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery undertaken by Xing et al. is another example of the need for further research on this theme.66 Nevertheless, we consider that levosimendan is likely associated at least with lower mortality rates than dobutamine, perhaps due, in part, to its more favourable impact on myocardial cellular energy balance. We further see no a priori reason that any treatment benefits identified in non-Chinese patients will not extend to Chinese patients. However, we note that aside from any consideration of ethnic differences, mortality and rehospitalisation rates among patients with decompensated HF remain high for some time after the index admission.
Safety Profile
Safety and tolerability concerns associated with levosimendan are well characterised, expected by its mode of action and, for the most part, demonstrated to be avoidable with due attention to issues such as baseline BP and fluid and electrolyte status. In fact, it has been recognised that hypotension can be a limitation of levosimendan applications, and several reports have suggested that omission of the loading dose of levosimendan in patients with low baseline BP or at risk of hypovolaemia can be a way to limit the incidence of hypotension (e.g. see the expert consensus by Nieminen et al.).47
In this area, the research of Gong et al. is of note.36 It is rare for adverse events or tolerability issues to necessitate cessation of levosimendan therapy. We see no evidence or theoretical reason to expect a different response from Chinese patients, but continued surveillance is warranted.
Conclusion
In cases of refractory HF, the (limited) experience reported by Chinese clinical researchers supports the expectation from studies elsewhere that levosimendan will deliver short-term improvements in haemodynamics and clinical symptomatic status. The preliminary work of Bonios et al. encourages an expectation that prolonged levosimendan therapy, administered as a succession of intermittent infusions, may also be accompanied by survival advantages in advanced HF refractory to conventional therapies.67 Indeed, a meta-analysis confirmed the association of repetitive levosimendan administration in advanced heart failure with a significant reduction in mortality at the longest follow-up available.68 However, confirmation of that finding is needed for patients of all ethnicities.
A recent report by Wang and Luo offers assurance on the use of levosimendan in patents with refractory HF accompanied by hypotension.69
Additional AHF scenarios that may warrant the use of levosimendan include decompensation accompanied by worsening renal function, cardiac ischaemia or elevated pulmonary artery pressure, as well as cardiogenic shock or takotsubo syndrome.70,71 To the extent that experience with levosimendan in HF in the context of AMI or acute decompensation or chronic or refractory HF has been substantially similar in Chinese and non-Chinese patients, we are encouraged to the view that the effects of levosimendan demonstrated in these additional clinical scenarios in predominantly non-Chinese populations are likely to be replicated in Chinese patients with otherwise similar clinical presentations. However, for all these additional indications it will be necessary to substantiate these expectations through direct study of Chinese patients in controlled studies.
For similar reasons, in this review we did not touch the perioperative use of the drug or its use in intensive care unit settings.72,73 Instead, we focused on its main indications in acute heart failure and acute cardiac care, as recently defined in the reviews by Farmakis et al. and Heringlake et al.74,75
From the data reviewed here, we conclude that there is enough evidence to show that the effects of levosimendan in acute and decompensated HF documented by studies in non-Chinese patients are replicated in Chinese patients and that experience accumulated by studies performed outside China may be regarded as a reasonable guide to the use of levosimendan in China.