Women have for many years been regarded as being at relatively low risk for the development of ischaemic heart disease (IHD).1,2 Recently this pattern has changed and cardiovascular disease (CVD) is now the leading cause of death for women in Europe.3,4 Under the age of 60 years acute coronary syndrome occurs 3–4 times more often in men; however, after the age of 75 years women represent the majority of the patients. Although coronary artery disease (CAD) develops on average 10 years later in women, CAD is responsible for approximately 20 % of deaths in both men and women.5,6 In regard to mortality, due to CVD women surpass men at 51 versus 42 %7,8 and over the last decades CVD mortality has declined rapidly for men, while the decline has been significantly lower for women.9,10
Chest Pain in Women
The typical symptom of CAD is oppressive chest pain radiating to the left arm. Nevertheless, the literature often notes that women tend to present with ’atypical’ symptoms: pain occurring at rest, during sleep or atypically located in the neck and shoulder regions, or fatigue, emotional stress, dyspnoea and general weakness.10,11 Studies have shown that women with characteristic symptoms have a higher likelihood of significant CAD.11,12 This suggests that the main difference between men and women is not so much the presentation of symptoms, but the high proportion of women with angina who have no obstructive CAD. In some studies this proportion has reached 70 % or more.13 Overall, treatment of stable angina pectoris is guided by the presence of obstructive CAD, which leaves patients with angina and no obstructive CAD under-diagnosed with few or no treatment options.1
Prognosis for Women with Angina and No Obstructive Coronary Artery Disease
Women with chest pain and no obstructive CAD were thought to have a benign prognosis, but contrary to previous beliefs, more women than expected may have a cardiac origin to their symptoms. Recent studies have found the condition to be associated with increased cardiovascular (CV) morbidity and mortality, and with a prognosis almost as poor as for subjects with obstructive CAD.13–15 In a study of 4,711 women with stable angina pectoris referred for coronary angiography (CAG) and 3,326 asymptomatic women, the hazard ratio for major CV events (MACE) in women with angina and normal coronary arteries and diffuse non-obstructive stenosis was 1.52 and 1.85, respectively, compared with asymptomatic women. The increased risk was comparable to patients with obstructive CAD and primarily due to CV death, heart failure or myocardial infarction.13 Similarly, the Women’s Ischemia Syndrome Evaluation (WISE) trial demonstrated higher prevalence of CVD events in 540 symptomatic women without CAD compared with 1,000 asymptomatic age- and race-matched women.15 Furthermore, the prognosis might be poorer for women compared with men. A large study (n=13,695) investigated sex differences in outcome after CAG in patients with angina and diffuse non-obstructive CAD and found that women were three-times more likely to experience a cardiac event within the first year compared with men.16
Coronary Microvascular Dysfunction
The pathophysiology behind angina with no obstructive CAD remains unknown. Coronary microvascular dysfunction (CMD) has been proposed as a possible explanation for the continued symptoms and higher CV morbidity and mortality. CMD is an impaired coronary microcirculatory function. Whereas epicardial coronary arteries are conduit vessels, the microcirculation consists of resistance vessels that regulate myocardial blood flow by dilation or contraction according to cardiac demand.17 A dysfunction of the resistance vessels reduces the capability to regulate myocardial perfusion leading to a decreased blood flow, stress-induced ischaemia and altered myocardial metabolism.18–20 The regulation of myocardial perfusion is complex and can be controlled via endothelial-dependent pathways by shear stress and endothelial-independent pathways, including regulation by metabolic substances, auto-regulation, myogenic control in response to increased intraluminal pressure and sympathetic nerve responses.21,22
CMD has multiple pathophysiologic pathways. Vascular causes include functional causes such as endothelial and smooth muscle dysfunction as well as structural causes such as microvascular atherosclerosis, intraluminal microembolism and vascular wall hypertrophy creating stiffness. Extravascular causes might also be a part of the pathogenesis, such as inflammation, structural changes such as ventricular hypertrophy causing vascular compression and impairment of the pathways regulating blood flow, i.e. imbalance of autonomic system with an inappropriate high sympathetic tone.23–25 Presumably, CMD occurs due to a synergy of many causes and it is difficult to isolate and identify the sole responsible cause.
Whether CMD explains both symptoms and the increased CVD risk in patients with angina and no obstructive CAD remains elusive due to diagnostic difficulties and lack of evidence-based intervention.
Diagnostic Methods to Assess Coronary Microvascular Dysfunction
A single method may not be sufficient to rule out CMD since each method may assess different aspects of the pathophysiology.26
Vasodilator Stimuli
Myocardial perfusion can be examined by using various vasodilator stimuli. Administration of adenosine, A1 –A3 receptor agonists, leads to coronary microvascular dilatation by acting directly on the vascular smooth muscle cells. Dipyridamole inhibits reuptake of adenosine and has a similar effect.27,28 Like adenosine, the selective A2a receptor agonist, regadenoson, dilates the coronary and peripheral arterial microvessels by acting on the smooth muscle cells.28 These vasodilators assess endothelial-independent coronary microvascular function27 in the absence of epicardial coronary artery stenosis.29 Microvascular function is expressed as a flow reserve calculated as the ratio between flow during stress and rest. Although CMD should be seen as a continuum,30 European guidelines suggest a flow reserve <2 as a strong indicator of CMD,31 but studies have used both higher and lower cut-off values depending on the method of assessment.19,20,32–34
Endothelium-dependent dysfunction and both macrovascular and microvascular spasm can be assessed by an intracoronary acetylcholine provocation test. 35 Vascular response to acetylcholine is predominantly caused by the release of nitric oxide (NO) from endothelial cells causing vasodilation.36 In patients with vascular dysfunction, examination with infusion of acetylcholine shows a diminished vasodilation, a paradoxical vasoconstriction or in larger doses coronary artery spasms may be elicited. This reaction has been suggested as part of the definition of vasospastic angina.37 The cold pressor test, the submersion of the patient’s hand in ice water, is also used to assess endothelial-dependent function. This procedure induces sympathetic stimulation, thereby increased heart rate, blood pressure and blood flow. The proposed mechanism is through adrenergic receptors releasing NO.38 However, for both acetylcholine and cold pressor tests, a direct effect on smooth muscle cells cannot be excluded and the response is not solely endothelial dependent.39
Invasive Assessment by Angiography
Epicardial blood flow can be measured by intracoronary Doppler flow where the tip of the guidewire has ultrasound capabilities measuring resting and peak velocities at maximal hyperaemia. To investigate resistance changes in the distribution of the epicardial coronary arteries, coronary vascular resistance can be calculated as mean arterial pressure divided by coronary blood flow.40 Additionally, quantitative vasomotor response in epicardial coronary arteries can be evaluated as a change in vessel diameter. Measurements are usually repeated using increasing concentrations of acetylcholine. Endothelial dysfunction is defined as vasoconstriction, lack of coronary blood flow increase and/or vasospasm as a result of direct activation of muscarinic receptors on vascular smooth muscle cells.37,41
Endothelial-independent function can also be assessed during vasodilator infusion with adenosine, dipyridamole or regadenoson with calculation of the coronary flow velocity reserve (CFVR).29 Flow velocity changes in the coronary arteries are assumed to be representative of changes in flow volume due to dilation of the smaller resistance vessels, based on the assumption that coronary artery diameters remain constant during hyperaemia. However, dilation of the epicardial coronary arteries during hyperaemia may occur. Therefore, intracoronary NO to achieve maximum dilation of the epicardial coronary arteries before CFVR measurement can be used as well as quantification of the cross-sectional area of the measuring point in the coronary artery calculating volumetric blood flow.42 Invasive CFVR measurement has been extensively used and validated and is at present considered the gold standard for assessment of endothelial-independent CMD.
Corrected thrombolysis in myocardial infarction (TIMI) frame counts can also be used as an invasive measure of CMD. The ratio between number of angiographic frames for the contrast to reach a distal landmark at maximal hyperaemia and rest reflects coronary microvascular function and has shown good correlation with invasive CFVR.29,43,44 CMD may also be assessed through thermodilution, although this method is less widely used.45
Positron Emission Tomography
Positron emission tomography (PET) can assess absolute myocardial blood flow (ml/min/g) by a dynamic imaging protocol calculating the rate of tracer uptake into the myocardium at rest and during stress.46 Rubidium (Rb)-82, oxygen (O)-water-15 and nitrogen (N)-13-ammonia are the most widely used tracers. Focusing on endothelial-dependent function, cold pressor testing can be used to induce stress by sympathetic stimulation calculating the absolute flow difference between rest and stress.34,47 The use of endothelial-independent vasodilators allows for calculation of the myocardial blood flow reserve (MBFR) for the assessment of coronary microvascular function.34,48 The few studies investigating the repeatability of MBFR have not been able to produce convincing results.49–51 However, one study found PET MBFR valid against invasively measured CFVR calculated by the volumetric blood flow method, where the cross-sectional area of the coronary artery was quantified to calculate blood flow volume.42
Echocardiographic Assessment
Transthoracic Doppler echocardiography (TTDE) can visualise the left anterior descending artery and peak coronary flow velocities at rest and maximum hyperaemia during vasodilator infusion with adenosine, dipyridamole or regadenoson can be measured by pulsed wave Doppler technique. The resulting CFVR reflects coronary microvascular function but may be affected by the possible dilation of the epicardial coronary arteries. The method can be technically challenging, but is highly reproducible in experienced hands with good agreement with invasively measured CFVR.52–58 However, agreement with PET measured MBFR is only modest in both women with no obstructive CAD (n=107) and patients with CAD (n=35).26,57 Advantages of the TTDE assessment is that it is easily accessible, non-invasive and radiation-free.59 Another method to assess CMD is myocardial perfusion echocardiography.60
Cardiac Magnetic Resonance
The microvasculature can be assessed by cardiac magnetic resonance (CMR) perfusion with images acquired immediately after the injection of gadolinium contrast.61 Contrast signal intensity gives an estimate of myocardial perfusion. The myocardial perfusion reserve index is the ratio of contrast signal intensity/upslope during pharmacologically induced hyperaemia and at rest. Use of CMR perfusion imaging in diagnosing CMD has not yet been standardised but having in mind that perfusion defects in CAD are visible even before angina, it is likely that women with CMD in the absence of obstructive CAD could have inducible perfusion abnormalities detectable by CMR. The reproducibility of perfusion CMR has been assessed in healthy volunteers with a coefficient of variation of 13.7 % for resting values and 12.9 % for stress values. Furthermore, inter- and intra-observer agreement was good.62 In patients with obstructive CAD, perfusion CMR correlated significantly with CFVR by Doppler flow guidewire.63
Computed Tomography Angiography
Computer tomography (CT) perfusion is a rapidly developing noninvasive diagnostic test evaluating the haemodynamic significance of coronary artery stenosis.64 Studies are currently establishing whether semi-quantitative measures, including the myocardial perfusion reserve (hyperaemic versus resting myocardial blood flow) and transmural perfusion ratio (subendocardial to subepicardial attenuation density) can identify patients with CMD.
The Coronary Microvascular Dysfunction Diagnosis
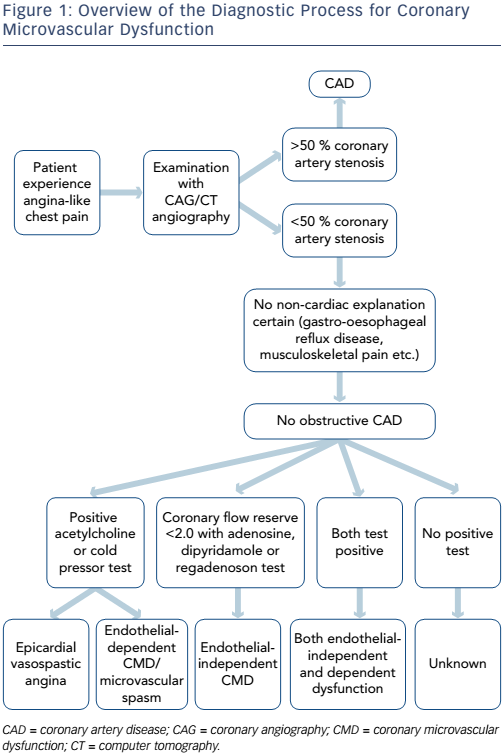
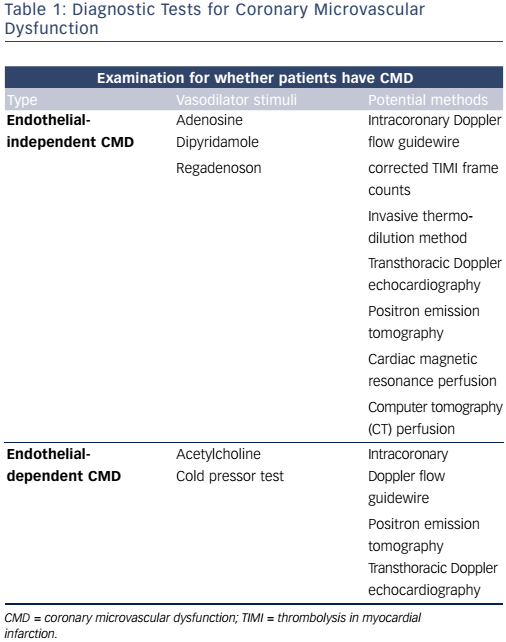
In summary, patients with no obstructive CAD can be diagnosed with either endothelial-dependent and/or endothelial-independent CMD depending on the choice of vasodilator using several methods (see Figure 1 and Table 1). However, there are multiple methods assessing coronary microvascular function and some seem to diverge and may be assessing different aspects of the complex pathophysiological mechanisms of both the endothelial independent and dependent part of CMD.26
Is Coronary Microvascular Dysfunction the Cause of Angina in Women with No Obstructive Coronary Artery Disease?
CMD is highly prevalent in a population of women and men with angina and no obstructive CAD. In a cross-sectional study of 159 women with no obstructive CAD examined invasively with CFVR, 47 % had CMD (CFVR <2.5).65 Another cross-sectional study of 1,439 patients with angina and no obstructive CAD found that 1,171 (81 %) had CMD with angiographic testing and infusion of both acetylcholine and adenosine: 19 % had both endothelial dependent and independent CMD; 12 % only endothelial-independent CMD and 33 % only endothelial-dependent CMD.66 In prospective and cross-sectional TTDE studies, CMD (CFVR <2.0) prevalence has been reported lower ranging from 22 to 40 %.33,67,68 In a prospective study of 1,218 patients assessed by PET more than 50 % of women were diagnosed as having endothelial-independent CMD (MBFR <2.0).69 Focusing on endothelial-dependent function, a prospective study of 470 patients with angina and no obstructive CAD found CMD defined as ≤50 % increase in myocardial blood flow to acetylcholine measured angiographically to be present in 56 %.70 Similarly 95 out of 147 patients (66 %) had CMD in a prospective study using >20 % diameter reduction in response to acetylcholine measured angiographically as criterion for CMD.71
Classification of angina symptoms, frequency, stability and treatment satisfaction were not associated with CMD assessed by TTDE CFVR in a cohort study of 919 women with angina. Burden of symptoms in terms of physical limitation and self-perception of disease was related to CMD, although differences between CFVR level were small.68 In accordance, PET MBFR (study n=1,218) was not different for patients with typical or atypical angina, compared with symptoms classified as non-cardiac or no symptoms.69 The WISE study compared CMR perfusion in women with angina and no obstructive CAD (n=53) to healthy controls (n=12) and found that the symptomatic women had a significantly lower myocardial perfusion reserve index but the burden of risk factors was also lower in the controls.72 Furthermore, trials (n=51 and n=46) have failed to show that improvement of symptoms are related to changes in coronary microvascular function.73,74 This questions whether CMD and the burden of symptoms are actually related; although symptoms are generally hard to study being indeed subjective.
Cardiovascular Risk Factors in Women with No Obstructive Coronary Artery Disease
A proportion of patients with angina and no obstructive CAD have IHD caused by insignificant atherosclerosis, vasospastic angina or CMD. Prevalence of CV risk factors in women with angina and no obstructive CAD is relatively high compared with the general population of women at similar ages and comparable to patients with coronary heart disease13,65,68,75 and independently predicts cardiovascular mortality.76,77 In recent large studies from the US and Denmark, approximately 50 % of women with angina and no obstructive CAD had hypertension, hyperlipidaemia and a family history of CAD, one-fifth had diabetes mellitus and generally these women had a high body mass index (BMI). However, active smoking varied from 16 %, 20 % and 30 % probably due to a general decline in smoking in the recent years.13,65,68 In comparison, around 35 % women in a Danish general population had hypertension, 8 % had diabetes and 20 % were actively smoking in the year 2013.75 CMD has been found to be associated with CV risk factors such as age,32,33,65,67–69,78,79 hypertension,33,68,69,79,80 diabetes mellitus,68,69,79,80 smoking,68,78 heart rate,68,81 HDL cholesterol,68,78 BMI,69,81 menopause and absence of hormone therapy post-menopause.65 However, not all studies identify the same CV risk factors and the contribution of risk factors explaining variation in CFR assessing CMD is low.66,68,82
The fact that prevalence of CV risk factors is high in women with angina and no obstructive CAD may play a role in the increased risk of CV events and possible CMD in this population.13,65,68,76
Signs of Myocardial Affection/Ischaemia
CMD is thought to be global or to involve random patchy areas of hypoxia, which might lead to transient myocardial ischaemia.83
Cardiac Exercise Stress Testing
Even in the absence of CAD, a large percentage of patients with persistent chest pain present with transient ischaemia on electrocardiogram due to regional myocardial hypoperfusion.18 However, ST-segment depression during an exercise test is thought to be less accurate in identifying CAD in women compared with men.84 Presence of impaired CFVR and endothelial dysfunction consistent with CMD is a plausible explanation for perfusion abnormalities sufficient to produce electrocardiogram changes and angina during stress testing. However, two large studies found no association between presence of CMD and results from diagnostic stress testing prior to CAG in women with angina and no obstructive CAD.66,68 The explanation may lie in the size and the possible patchy distribution of the affected, ischaemic myocardium, undetectable by present techniques in cardiac stress testing. Decreased exercise tolerance, vasospastic angina and oestrogen levels have also been suggested as causes for ST-depression in women with no obstructive CAD.84,85
Markers of Inflammation
Chronic inflammation, expressed as elevated C-reactive protein levels, has been found associated with endothelial dysfunction and CMD in patients with no obstructive CAD.86,87 Patients with angina and a normal CAG were in one study (n=137) found to have elevated C-reactive protein levels and reduced PET measured MBFR compared with controls.88
Prognosis for Patients with Coronary Microvascular Dysfunction
Epicardial and microvascular coronary endothelial dysfunction assessed invasively by acetylcholine predict long-term risk of CV events in patients with no obstructive CAD (study n between 147 and 470), independently of traditional risk factors.70,71,89,90 In a study of72, cold pressor testing in PET imaging can be used to predict CV outcome.47
Endothelial-independent CMD assessed both invasively (n=152) by PET (n=1,218) and by Doppler echocardiography (n=394) has been shown to be strongly associated with adverse CV outcome in several prospective studies of patients with no obstructive CAD.32,33,69
Large prognostic studies of perfusion CMR in patients without obstructive CAD are lacking, but in one study of 103 patients with angina and normal stress perfusion CMR no patient with a normal CMR experienced events, whereas 16 events occurred in 14 patients with abnormal CMR during follow-up.91 In the WISE study, global perfusion abnormalities reflecting CMD could also predict adverse events.92
Continued Symptoms
Patients with angina pectoris and no obstructive CAD are costly for society due to continued symptoms resulting in multiple hospital re-admissions and re-assessments for obstructive CAD.93 Angina is persistent in 45–61 % of patients after 1–3 years.94–97 Persistent angina has been reported to be associated with non-fatal myocardial infarction and stroke.95 Frequent angina was furthermore found to be associated with lower physical functioning, angina stability, treatment satisfaction and quality of life.94 Patients with angina and no obstructive CAD have increased risk of hospitalisation primarily due to stable and unstable angina, repeat CAG, heart failure and stroke compared with healthy controls. Furthermore, higher rates of followup consultations at the general practitioners have been reported.93,98
Besides high healthcare costs, patients with angina and no obstructive CAD often exit the workforce prematurely requiring disability pension. Surprisingly, costs are comparable to patients with obstructive CAD.97,99 Cumulative costs over a five-year period has, in an American study, been estimated to be lower, though still very high, for women with no obstructive CAD (US$32,239; 95 % confidence interval [CI], US$29,496–US$34,982) than for patients with obstructive CAD (increasing to US$53,398; 95 % CI, US$43,854–US$62,942 for patients with three-vessel disease).93
Treatment Strategies
Currently, there are no evidence-based treatment strategies for women with no obstructive CAD. However, guideline-based management of the relative high burden of risk factors in all patients with IHD, including women with no obstructive CAD, is important. Further approaches are lifestyle modification (smoking cessation, exercise and a healthy diet).100 Moreover, large clinical trials are lacking for those diagnosed with any aspect of CMD. Small clinical trials indicate that anti-ischaemic and antianginal medication (such as treatment with angiotensin-converting enzyme inhibitor, statins and NO modulators) could have a role in improving CMD. However, the trials conducted have been of moderate or poor quality and/or with too few subjects (n from 6 to 13) to have an impact on guidelines.24
Closer to a Diagnosis?
Women with angina pectoris and no obstructive CAD have continued symptoms and a poor CV prognosis. CMD is an entity comprising several pathologic pathways. New invasive and non-invasive diagnostic modalities for identifying CMD are being developed and accumulating research points to CMD as a cause of poor prognosis in women with angina and no obstructive CAD. Whether CMD directly causes angina cannot be decisively concluded at present, for this we must await intervention studies showing simultaneous improvement in CMD and angina symptoms. Furthermore, evidence-based treatment strategies for women with no obstructive CAD are lacking.