Abstract
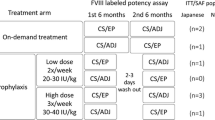
The safety and efficacy of Kogenate, a recombinant factor VIII (rFVIII) preparation for the treatment of bleeding episodes, were studied in a 123-patient meta-analysis population of previously treated patients (PTPs), including 15 enrolled in the registration Phase III trial (PTP-I group), 93 from the post-marketing special investigation (PTP-II group), and 15 from short-term special investigations in surgery or tooth extraction (SI group). These patients (82 severe, 31 moderate, 9 mild, and 1 unknown), aged 11 months to 72 years, were enrolled in 28 centers in Japan. Blood samples taken at the baseline and at 3, 6, 9, 12, 18, and 24 months after the introduction of Kogenate were evaluated for FVIII inhibitor antibodies, antibodies formed against trace proteins derived from the rFVIII production process, and for general changes in laboratory test results. Mean exposure to Kogenate was 1103 days in PTP-I, 86 days in PTP-II, 27 days in patients in surgery, and 2 days in patients with tooth extraction.
Assessment of FVIII inhibitor activity was conducted in 115 of the 123 patients by means of the Bethesda assay. Twelve patients were found to have a low titer of FVIII inhibitor (0.5–3.0 BU/mL) prior to any administration of Kogenate, and 103 were inhibitor-negative at the baseline. Among this latter group, 3 patients (2.9%) tested inhibitor-positive, with titers ranging from 1.2 to 2.1 BU/mL, with 4 patients below 1.0 BU/mL. One patient in the 11 PTPs investigated (PTP-I) developed antibodies against baby hamster kidney protein and mouse immunoglobulin G, but these findings were transient and asymptomatic. Hemostasis was achieved (markedly effective or effective) in 3666 of the 3855 bleeding episodes (95.1%) observed in 108 patients. Only 1 infusion was necessary in 3790 (98.3%) of these episodes. These data indicate that Kogenate is safe and very effective for the treatment of bleeding in PTPs with hemophilia A.
Similar content being viewed by others
References
Gitschier J, Wood WI, Goralka TM, et al. Characterization of the human factor VIII gene. Nature. 1984;312:326–330.
Wood WI, Capon DJ, Simonsen CC, et al. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984;312:330–337.
Schwartz RS, Abildgaard CF, Aledort LM, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A: Recombinant Factor VIII Study Group. N Engl J Med. 1990;323:1800–1805.
Yoshioka A. Advances in hemophilia and other coagulation disorders: recombinant factor VIII preparations. Int J Pediatr Hematol Oncol. 1994;1:491–497.
Fukui H,Yoshioka A, Shima M, et al. Clinical evaluation of recombinant human factor VIII (BAY w 6240) in the treatment of hemophilia A. Int J Hematol. 1991;54:419–427.
Fukui H, Mori K, Ishikawa M, et al. A long-term, multicenter clinical study of recombinant human factor VIII (BAY w 6240) in the treatment of hemophilia A [in Japanese]. Nihon Yuketsu Gakkai Zasshi. 1991;37:593–604.
Yoshioka A, Fukutake K, Takamatsu J, et al. Clinical evaluation of a recombinant factor VIII preparation (Kogenate) in previously untreated patients with hemophilia A. Int J Hematol. 2003;78:467–474.
Kasper CK, Aledort LM, Counts RB, et al. A more uniform measurement of factor VIII inhibitors [letter]. Thromb Diath Haemorrh. 1975;34:869–872.
Shima M, Sawamoto Y, Nakai H, et al. Measurement of anti-factor VIII IgG, IgG4 and IgM alloantibodies in previously untreated hemophilia A patients treated with recombinant factor VIII: Kogenate Japanese Clinical Study Group. Int J Hematol. 1995;62:35–43.
Ehrenforth S, Kruetz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet. 1992;339:594–598.
Addiego J, Kasper C, Abildgaard C, et al. Frequency of inhibitor development in haemophiliacs treated with low-purity factor VIII. Lancet. 1993;342:462–464.
Ljung R, Petrini P, Lindgren A, et al. Factor VIII and factor IX inhibitor in haemophiliacs. Lancet. 1992;339:1550–1551.
deBiasi R, Rocino A, Papa ML, et al. Incidence of factor VIII inhibitor development in hemophilia A patients treated with less pure plasma derived concentrates. Thromb Haemost. 1994;71:544–547.
Schwartz RS, Abildgaard CF, Aledort LM, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of haemophilia A. N Engl J Med. 1990;323:1800–1805.
Bray GL, Gomperts ED, Courter S, et al. A multicenter study of recombinant factor VIII (Recombinate): safety, efficacy and inhibitor risk in previously untreated individuals with hemophilia A. Blood. 1994;83:2428–2435.
Lusher JM, Spira J, Rodriguez D. A four-year update of safety and efficacy of an albumin-free formulated B-domain deleted factor VIII (BDD rFVIII, rVIIISQ) in previously untreated severe hemophilia A patients. Thromb Haemost. 1999;82:1493.
Abe T, Igata A, Ikematsu S, et al. Multicenter clinical effect of heat-treated factor VIII (CP-8) preparation for patients with hemophilia A [in Japanese]. Rinshio to Kenkyu. 1985;62:3640–3659.
Fujimaki M, Tanaka A, Fukutake K, et al. Clinical evaluation of recombinant factor VIII (BL-160) in patients with hemophilia A [in Japanese]. Nihon Kessen Shiketsu Gakkaishi. 1993;4:179–188.
Fujimaki M, Goto M, Miyazaki T, et al. Clinical study of FVIII concentrate highly purified by monoclonal antibodies to FVIII(RCG- 11) [in Japanese]. Kiso to Rinsho. 1992;26:299–319.
McMillan C, Shapiro S, Whitehurst D, et al. The natural history of factor VIII C inhibitors in patients with hemophilia A. A national cooperative study II. Observations on the initial development of factor VIII C inhibitors. Blood. 1988;71:344–348.
White GC, Courter S, Bray GL, et al. A multicenter study of recombinant factor VIII (RecombinateH) in previously treated patient with hemophilia A. Thromb Haemost. 1997;77:660–667.
Seremetis S, Lusher JM,Abildgaard CF, et al. Human recombinant DNA-derived antihaemophilic factor (factor VIII) in the treatment of haemophilia A: conclusions of a 5-year study of home therapy. Haemophilia. 1999;5:9–16.
Lusher JM, Lee CA, Kessler CM, Bedrosian CL, for the ReFacto Phase 3 Study Group. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9:38–49.
Giles AR, Rivard GE, Teitel J, Walker I. Surveillance for factor VIII inhibitor development in the Canadian hemophilia A population following the widespread introduction of recombinant factor VIII replacement therapy. Transfus Sci. 1998;19:139–48.
Smith MP, Giangrande P, Pollman H, et al. A postmarketing surveillance study of safety and efficacy of Refacto (St. Louis-derived active substance) in patients with haemophilia. Haemophilia. 2005;11:444–451.
Rosendaal FR, Nieuwenhuis HK, van den Berg HM, et al. A sudden increase in factor VIII inhibitor development in multitransfused hemophilia A patients in the Netherlands. Blood. 1993;81:2180–2186.
Giles AR, Verbruggen B, Rivard GE, Teitel J, Walker I. A detailed comparison of the performance of the standard versus the Nijmegen modification of the Bethesda assay in detecting factor VIII:C inhibitors in the haemophilia A population of Canada. Association of Hemophilia Centre Directors of Canada. Factor VIII/IX Subcommittee of Scientific and Standardization Committee of International Society on Thrombosis and Haemostasis. Thromb Haemost. 1998;79:872–875.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Yoshioka, A., Fukutake, K., Takamatsu, J. et al. Clinical Evaluation of Recombinant Factor VIII Preparation (Kogenate) in Previously Treated Patients with Hemophilia A: Descriptive Meta-Analysis of Post-Marketing Study Data. Int J Hematol 84, 158–165 (2006). https://doi.org/10.1532/IJH97.06019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1532/IJH97.06019