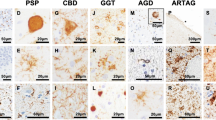
Abstract
The microtubule (MT)-associated protein (MAP) tau in neurons has been implicated as a significant factor in the axonal growth, development of neuronal polarity, and the maintenance of MT dynamics. Tau is localized to the axon, and is known to promote MT assembly and to stabilize axonal MTs. These functions of tau are primarily regulated by the activities of protein kinases and phosphatases. In Alzheimer’s disease and other neurodegenerative disorders, abundant filamentous tau inclusions are found to be major neuropathological characteristics of these diseases. Both somato-dendritic and axonal tau lesions appear to be closely associated with axonal disruption. Furthermore, recent discoveries of pathogenic mutations on the tau gene suggest that abnormalities of tau alone are causative of neurodegeneration. Finally, analyses of transgenic mice that express human tau proteins have enabled in vivo quantitative assessments of axonal functions and have provided information about mechanistic relationships between pathological alteration of tau and axonal degeneration.
Similar content being viewed by others
References
Ahlijanian M. K., Barrezueta N. X., Williams R. D., Jakowski A., Kowsz K. P., et al. (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc. Natl. Acad. Sci. USA 97(6), 2910–2915.
Andreadis A., Brown W. M., and Kosik K. S. (1992) Structure and novel exons of the human tau gene. Biochemistry 31(43), 10,626–10,633.
Arrasate M., Perez M., Armas-Portela R., and Avila J. (1999) Polymerization of tau peptides into fibrillar structures. The effect of FTDP-17 mutations. FEBS Lett. 446(1), 199–202.
Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., and Hyman B. T. (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42(3 Pt 1), 631–639.
Auer I. A., Schmidt M. L., Lee V. M.-Y., et al. (1995) Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer’s disease. Acta Neuropathol. (Berl) 90, 547–551.
Baas P. W., Deitch J. S., Black M. M., and Banker G. A. (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA 85(21), 8335–8339.
Bancher C., Lassmann H., Budka H., et al. (1987) Neurofibrillary tangles in Alzheimer’s disease and progressive supranuclear palsy: antigenic similarities and differences. Micortubule-associated protein tau antigenicity is prominent in all types of tangles. Acta Neuropathol. (Berl) 74, 39–46.
Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., et al. (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 477(1–2), 90–99.
Bancher C., Braak H., Fischer P., and Jellinger K. A. (1993) Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci. Lett. 162(1–2), 179–182.
Bancher C., Jellinger K., Lassmann H., Fischer P., and Leblhuber F. (1996) Correlations between mental state and quantitative neuropathology in the Vienna Longitudinal Study on Dementia. Eur. Arch. Psychiatry Clin. Neurosci. 246(3), 137–146.
Barghorn S., Zheng-Fischhöfer Q., Ackmann M., Biernat J., von Bergen M., et al. (2000) Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry 39(38), 11,714–11,721.
Baudier J. and Cole R. D. (1987) Phosphorylation of tau proteins to a state like that in Alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J. Biol. Chem. 262(36), 17,577–17,583.
Baumann K., Mandelkow E. M., Biernat J., Piwnica-Worms H., and Mandelkow E. (1993) Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 336(3), 417–424.
Bernhardt R. and Matus A. (1984) Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J. Comp. Neurol. 226(2), 203–221.
Biernat J., Gustke N., Drewes G., Mandelkow E. M., and Mandelkow E. (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immuno-reactivity and microtubule binding. Neuron 11(1), 153–163.
Billingsley M. L. and Kincaid R. L. (1997) Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 323 (Pt 3), 577–591.
Binder L. I., Frankfurter A., and Rebhun L. I. (1985) The distribution of tau in the mammalian central nervous system. J. Cell Biol. 101(4), 1371–1378.
Bobinski M., Wegiel J., Tarnawski M., Bobinski M., Reisberg B., de Leon M. J., et al. (1997) Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J. Neuropathol. Exp. Neurol. 56(4), 414–420.
Braak H. and Braak E. (1987) Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci. Lett. 76, 124–127.
Braak H. and Braak E. (1992) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16(3), 271–278, discussion 278–284.
Braak E., Braak H., and Mandelkow E. M. (1994) A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. (Berl) 87(6), 554–567.
Brady S. T., Witt A. S., Kirkpatrick L. L., de Waegh S. M., Readhead C., Tu P. H., et al. (1999) Formation of compact myelin is required for maturation of the axonal cytoskeleton. J. Neurosci. 19(17), 7278–7288.
Bramblett G.T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., and Lee V. M.-Y. (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10(6), 1089–1099.
Brandt R., Leger J., and Lee G. (1995) Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J. Cell Biol. 131(5), 1327–1340.
Brion J. P., Guilleminot J., Couchie D., Flament D. J., and Nunez J. (1988) Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum. Neuroscience 25(1), 139–146.
Brion J. P., Tremp G., and Octave J. N. (1999) Transgenic expression of the shortest human tau affects its compartmentalization and its phosphorylation as in the pretangle stage of Alzheimer’s disease. Am. J. Pathol. 154(1), 255–270.
Buée L., Bussiere T., Buee-Scherrer V., Delacourte A., and Hof P. R. (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 33(1), 95–130.
Buée-Scherrer V., Buée L., Hof P. R., et al. (1995) Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: immunochemical characterization of tau proteins. Am. J. Pathol. 146, 924–932.
Bugiani O., Murrell J. R., Giaccone G., Hasegawa M., Ghigo G., et al. (1999) Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J. Neuropathol. Exp. Neurol. 58(6), 667–677.
Burton P. R. and Paige J. L. (1981) Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc. Natl. Acad. Sci. USA 78(5), 3269–3273.
Butner K. A. and Kirschner M. W. (1991) Tau protein binds to microtubules through a flexible array of distributed weak sites. J. Cell Biol. 115(3), 717–730.
Caceres A. and Kosik K. S. (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343(6257), 461–463.
Caceres A., Potrebic S., and Kosik K. S. (1991) The effect of tau antisense oligonucleotides on neurite formation of cultured cerebellar macroneurons. J. Neurosci. 11(6), 1515–1123.
Chen J., Kanai Y., Cowan N. J., and Hirokawa N. (1992) Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature 360(6405), 674–677.
Chin S. S.-M. and Goldman J. E. (1996) Glial inclusions in CNS degenerative diseases. J. Neuropathol. Exp. Neurol. 55(5), 499–508.
Clark L. N., Poorkaj P., Wszolek Z., Geschwind D. H., Nasreddine Z. S., et al. (1998) Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc. Natl. Acad. Sci. USA 95(22), 13,103–13,107.
Cleveland D. W., Hwo S.-Y., and Kirschner M. W. (1977) Purification of tau, a microtubule-associated protein that induces assembly of micro-tubules from purified tubulin. J. Mol. Biol. 116(2), 207–225.
Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123), 921–923.
Couchie D., Mavilia C., Georgieff I. S., Liem R. K., Shelanski M. L., and Nunez J. (1992) Primary structure of high molecular weight tau present in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 89(10), 4378–4381.
Cummings B. J. and Cotman C. W. (1995) Image analysis of beta-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet 346(8989), 1524–1528.
Cummings B. J., Pike C. J., Shankle R., and Cotman C. W. (1996) Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiol. Aging 17(6), 921–933.
Dawson H. N., Ferreira A., Eyster M. V., Ghoshal N., Binder L. I., and Vitek M. P. (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 114(Pt 6), 1179–1187.
De Camilli P., Miller P. E., Navone F., Theurkauf W. E., and Vallee R. B. (1984) Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience 11(4), 817–846.
de Waegh S. M., Lee V. M.-Y., and Brady S. T. (1992) Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 68, 451–463.
Dickson D. W., Crystal H. A., Mattiace L. A., Masur D. M., Blau A. D., Davies P., et al. (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging 13(1), 179–189.
Dickson D. W., Feany M. B., Yen S. H., Mattiace L. A., and Davies P. (1996) Cytoskeletal pathology in non-Alzheimer degenerative dementia: new lesions in diffuse Lewy body disease, Pick’s disease, and corticobasal degeneration. J. Neural. Transm. Suppl. 47, 31–46.
Dickson D. W. (1998) Pick’s disease: a modern approach. Brain Pathol. 8(2), 339–354.
Drechsel D. N., Hyman A. A., Cobb M. H., and Kirschner M. W. (1992) Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 3(10), 1141–1154.
Drewes G., Lichtenberg-Kraag B., Doring F., Mandelkow E. M., Biernat J., Goris J., et al. (1992) Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 11(6), 2131–2138.
Drewes G., Mandelkow E. M., Baumann K., Goris J., Merlevede W., and Mandelkow E. (1993) Dephosphorylation of tau protein and Alzheimer paired helical filaments by calcineurin and phosphatase-2A. FEBS Lett. 336(3), 425–432.
Drewes G., Ebneth A., Preuss U., Mandelkow E. M., and Mandelkow E. (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89(2), 297–308.
D’Souza I., Poorkaj P., Hong M., Nochlin D., Lee V. M.-Y., et al. (1999) Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 96(10), 5598–5603.
Duff K., Knight H., Refolo L. M., Sanders S., Yu X., Picciano M., et al. (2000) Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis. 7(2), 87–98.
Feany M. B. and Dickson D. W. (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am. J. Pathol. 146(6), 1388–1396.
Feany M. B., Mattiace L. A., and Dickson D. W. (1996) Neuropathologic overlap of progressive supra-nuclear palsy, Pick’s disease and corticobasal degeneration. J. Neuropathol. Exp. Neurol. 55(1), 53–67.
Flament S., Delacourte A., Verny M., et al. (1991) Abnormal tau proteins in progressive supranuclear palsy: similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol. (Berl) 81, 591–596.
Forman M. S., Lee V. M.-Y., and Trojanowski J. Q. (2000) New insights into genetic and molecular mechanisms of brain degeneration in tauopathies. J. Chem. Neuroanat. 20(3–4), 225–244.
Forman M. S., Zhukareva V., Bergeron C., Chin S. S., Grossman M., Clark C., et al. (2002) Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am. J. Pathol. 160(6), 2045–2053.
Foster N. L., Wilhelmsen K., Sima A. A., et al. (1997) Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference participants. Ann. Neurol. 41, 706–715.
Gamblin T. C., King M. E., Dawson H., Vitek M. P., Kuret J., et al. (2000) In vitro polymerization of tau protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry 39(20), 6136–6144.
Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., et al. (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373(6514), 523–527.
Ginsberg S. D., Galvin J. E., Chiu T. S., Lee V. M.-Y., Masliah E., and Trojanowski J. Q. (1998) RNA sequestration to pathological lesions of neurodegenerative diseases. Acta Neuropathol. 96(5), 487–494.
Goedert M., Wischik C. M., Crowther R. A., Walker J. E., and Klug A. (1988) Cloning and swquencing of the cDNA encoding a core protein of the paired helical filament of alzheimer disease: identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA 85(11), 4051–4055.
Goedert M., Spillantini M. G., Jakes R., Rutherford D., and Crowther R. A. (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3(4), 519–526.
Goedert M. and Jakes R. (1990) Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 9(13), 4225–4230.
Goedert M., Cohen E. S., Jakes R., and Cohen P. (1992a) p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer’s disease. FEBS Lett. 312(1), 95–99.
Goedert M., Spillantini M. G., Cairns N. J., and Crowther R. A. (1992b) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8(1), 159–168.
Goedert M., Jakes R., Crowther R. A., Six J., Lubke U., Vandermeeren M., et al. (1993) The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc. Natl. Acad. Sci. USA 90(11), 5066–5070.
Goedert M., Spillantini M.G., Jakes R., Crowther R. A., Vanmechelen E., Probst A., et al. (1995) Molecular dissection of the paired helical filament. Neurobiol. Aging 16(3), 325–334.
Goedert M., Jakes R., Spillantini M. G., Hasegawa M., Smith M. J., and Crowther R. A. (1996) Assembly of microtubule-associated protein tau into Alzheimer-like filaments induce by sulphated glycosaminoglycans. Nature 383(6600), 550–553.
Goedert M., Hasegawa M., Jakes R., Lawler S., Cuenda A., and Cohen P. (1997a) Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 409(1), 57–62.
Goedert M., Trojanowski J. Q., and Lee V.M.-Y. (1997b) The neurofibrillary pathology of Alzheimer’s disease, in The Molecular and Genetic Basis of Neurological Diseases, second ed. (Prusiner S. B., Rosenberg R. N., Di Mauro S., et al., eds.), Boston, MA, pp. 613–627.
Goedert M., Jakes R., and Crowther R. A. (1999) Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 450(3), 306–311.
Gomez-Isla T., Price J. L., McKeel D. W. Jr., Morris J. C., Growdon J. H., and Hyman B. T. (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 16(14), 4491–4500.
Goode B. L. and Feinstein S. C. (1994) Identification of a novel microtubule binding and assembly domain in the developmentally regulated interrepeat region of tau. J. Cell Biol. 124(5), 769–782.
Götz J., Probst A., Spillantini M. G., Schafer T., Jakes R., et al. (1995) Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 14(7), 1304–1313.
Götz J., Chen F., van Dorpe J., and Nitsch R. M. (2001) Formation of neurofibrillary tangles in P3011 tau transgenic mice induced by Abeta 42 fibrils. Science 293(5534), 1491–1495.
Greenberg S. G. and Davies P. (1990) A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc. Natl. Acad. Sci. USA 87(15), 5827–5831.
Greenberg S. G., Davies P., Schein J. D., and Binder L. I. (1992) Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J. Biol. Chem. 267(1), 564–569.
Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., and Binder L. I. (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 83(13), 4913–4917.
Gustke N., Steiner B., Mandelkow E. M., Biernat J., Meyer H. E., Goedert M., et al. (1992) The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 307(2), 199–205.
Hagestedt T., Lichtenberg B., Wille H., Mandelkow E. M., and Mandelkow E. (1989) Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J. Cell Biol. 109(4 Pt 1), 1643–1651.
Hanger D. P., Hughes K., Woodgett J. R., Brion J. P., and Anderton B. H. (1992) Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 147(1), 58–62.
Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., et al. (1994) Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369(6480), 488–491.
Harris K. A., Oyler G. A., Doolittle G. M., Vincent I., Lehman R. A., Kincaid R. L., et al. (1993) Okadaic acid induces hyperphosphorylated forms of tau protein in human brain slices. Ann. Neurol. 33(1), 77–87.
Hasegawa M., Jakes R., Crowther R. A., Lee V. M.-Y., Ihara Y., and Goedert M. (1996) Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 384(1), 25–30.
Hasegawa M., Crowther R. A., Jakes R., and Goedert M. (1997) Alzheimer-like changes in microtubule-associated protein tau induced by sulfated glycosaminoglycans. Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. J. Biol. Chem. 272(52), 33,118–33,124.
Hasegawa M., Smith M. J., and Goedert M. (1998) Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 437(3), 207–210.
Hasegawa M., Smith M. J., Iijima M., Tabira T., and Goedert M. (1999) FTDP-17 mutations N279K and S305N in tau produce increased splicing of exon 10. FEBS Lett. 443(2), 93–96.
Hauw J. J., Daniel S. E., Dickson D., Horoupian D. S., Jellinger K., et al. (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44(11), 2015–2019.
Heidemann S. R., Landers J. M., and Hamborg M. A. (1981) Polarity orientation of axonal microtubules. J. Cell Biol. 91(3 Pt 1), 661–665.
Higuchi M., Trojanowski J. Q., and Lee V. M.-Y. (2002) Tau protein and tauopathy, in Neuropsychopharmacology: The Fifth Generation of Progress (Davis K. L., Charney D., Coyle J., and Nemeroff C. N., eds.), Lippincott Williams & Wilkins, Baltimore, MD, pp. 1339–1353.
Himmler A., Drechsel D., Kirschner M. W., and Martin D. W. Jr. (1989) Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol. Cell Biol. 9(4), 1381–1388.
Hirano A., Malamud N., and Kurland L. T. (1961) Parkinsonism dementia complex in endemic disease on the island of Guam—pathologic features. Brain 84, 662.
Hirokawa N., Funakoshi T., Sato-Harada R., and Kanai Y. (1996) Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J. Cell Biol. 132(4), 667–679.
Hof P. R., Nimchinsky E. A., Buée-Scherrer V., et al. (1994) Amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: quantitative neuropathology, immunohistochemical analysis of neuronal vulnerability, and comparison with related neurodegenerative disorders. Acta Neuropathol. (Berl) 88, 397–404.
Hoffmann R., Lee V. M.-Y., Leight S., Varga I., and Otvos L. Jr. (1997) Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry 36(26), 8114–8124.
Hong M. and Lee V. M.-Y. (1997) Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 272(31), 19,547–19,553.
Hong M., Chen D. C., Klein P.S., and Lee V.M.-Y. (1997) Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 272(40), 25,326–25,332.
Hong M., Zhukareva V., Vogelsberg-Ragaglia V., Wszolek Z., Reed L., Miller B. I., et al. (1998) Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282(5395), 1914–1917.
Hosoi T., Uchiyama M., Okumura E., Saito T., Ishiguro K., Uchida T., et al. (1995) Evidence for cdk5 as a major activity phosphorylating tau protein in porcine brain extract. J. Biochem. (Tokyo) 117(4), 741–749.
Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., et al. (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274(5284), 99–102.
Hutton M., Lendon C. I., Rizzu P., Baker M., Froelich S., et al. (1998) Association of missense and 5′-spilice-site mutations in tau with the inherited dementia FTDP-17. Nature 393(6686), 702–705.
Hyman B. T., West H. L., Gomez-Isla T., and Mui S. (1995) Quantitative neuropathology in Alzheimer’s disease: Neuronal loss in high-order association cortex parallels dementia. In Research Advances in Alzheimer’s Disease and Related Disorders (Iqbal K., Mortimer J. A., Winblad B., and Wisniewski H. M., eds.), Wiley, New York, NY, pp. 453–460.
Iijima M., Tabira T., Poorkaj P., Schellenberg G. D., Trojanowski J. Q., et al. (1999) A distinct familial presenile dementia with a novel missense mutation in the tau gene. NeuroReport 10(3), 497–501.
Ikegami S., Harada A., and Hirokawa N. (2000) Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci Lett. 279(3), 129–132.
Ishiguro K., Takamatsu M., Tomizawa K., Omori A., Takahashi M., Arioka M., Uchida T., and Imahori K. (1992) Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J. Biol. Chem. 267(15), 10,897–10,901.
Ishihara T., Hong M., Zhang B., Nakagawa Y., Lee M. K., et al. (1999) Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 24(3), 751–762.
Ishihara T., Zhang B., Higuchi M., Yashiyama Y., Trojanowski J. Q., and Lee V. M.-Y. (2001) Age dependent induction of congophilic neurofibrillary inclusions in tau transgenic mice. Am. J. Pathol. 158(2), 555–562.
Ishihara T., Higuchi M., Zhang B., Yoshiyama Y., Hong M., Trojanowski J. Q., et al. (2001) Attenuated neurodegenerative disease phenotype in tau transgenic mouse lacking neurofilaments. J. Neurosci. 21(16), 6026–6035.
Itagaki S., McGeer P. L., Akiyama H., et al. (1989) A case of adult-onset dementia with argyrophilic grains. Ann. Neurol. 26, 685–689.
Iwatsubo T., Hasegawa M., and Ihara Y. (1994) Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol. 88(2), 129–136.
Kampers T., Friedhoff P., Biernat J., Mandelkow E. M., and Mandelkow E. (1996) RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett. 399(3), 344–349.
Kanemaru K., Takio K., Miura R., Titani K., and Ihara Y. (1992) Fetal-type phosphorylation of the tau in paired helical filaments. J. Neurochem. 58(5), 1667–1675.
Kidd M. (1963) Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197, 192–194.
Kirkpatrick L. L., Witt A. S., Payne H. R., Shine H. D., and Brady S. T. (2001) Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons. J. Neurosci. 21(7), 2288–2297.
Kobayashi S., Ishiguro K., Omori A., Takamatsu M., Arioka M., Imahori K., et al. (1993) A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30 kDa subunit of tau protein kinase II, a proline-directed protein kinase associated with microtubule. FEBS Lett. 335(2), 171–175.
Komori T., Arai N., Oda M., Nakayama H., Mori H., Yagishita S., et al. (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. (Berl) 96(4), 401–408.
Komori T. (1999) Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 9(4), 663–679.
Kondo J., Honda T., Mori H., Hamada Y., Miura R., Ogawara M., et al. (1988) The carboxyl third of tau is tightly bound to paired helical filaments. Neuron 1(9), 827–834.
Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M.-Y., and Lee G. (1988) Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1(9), 817–825.
Kosik K. S., Orecchio L. D., Bakalis S., and Neve R. L. (1989) Developmentally regulated expression of specific tau sequences. Neuron 2(4), 1389–1397.
Kowall N. W. and Kosik K. S. (1987) Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer’s disease. Ann. Neurol. 22(5), 639–643.
Ksiezak-Reding H., Morgan K., Mattiace L. A., et al. (1994) Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am. J. Pathol. 145, 1496–1508.
Lee G., Cowan N., and Kirschner M. W. (1988) The primary structure and heterogeneity of tau protein from mouse brain. Science 239(4837), 285–288.
Lee G., Neve R. L., and Kosik K. S. (1989) The microtubule binding domain of tau protein. Neuron 2(6), 1615–1624.
Lee V. M.-Y., Balin B. J., Otvos L. Jr., and Trojanowski J. Q. (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251(4994), 675–678.
Lee V. M.-Y., Goedert M., and Trojanowski J. Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159.
Leost M., Schultz C., Link A., Wu Y. Z., Biernat J., Mandelkow E. M., et al. (2000) Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 267(19), 5983–5994.
Lewis J., McGowan E., Rockwood J., Melrose H., Nacharaju P., et al. (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25(4), 402–405.
Lewis J., Dickson D. W., Lin W. L., Chisholm L., Corral A., Jones G., et al. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293(5534), 1487–1491.
Lieberman A. P., Trojanowski J. Q., Lee V. M.-Y., et al. (1998) Cognitive, neuroimaging, and pathological studies in a patient with Pick’s disease. Ann. Neurol. 43, 259–265.
Lindwall G. and Cole R. D. (19??) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259(8), 5301–5305.
Litersky J. M. and Johnson G. V. (1992) Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J. Biol. Chem. 267(3), 1563–1568.
Litman P., Barg J., Rindzoonski L., and Ginzburg I. (1993) Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron 10(4), 627–638.
Litvan I., Agid Y., Calne D., Campbell G., Dubois B., et al. (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1), 1–9.
LoPresti P., Szuchet S., Papasozomenos S. C., Zinkowski R. P., and Binder L. I. (1995) Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc. Natl. Acad. Sci. USA 92(22), 10,369–10,373.
Love S., Bridges L. R., and Case C. P. (1995) Neurofibrillary tangles in Niemann-Pick disease type C. Brain 188, 119–129.
Lovell M. A., Ehmann W. D., Mattson M. P., and Markesbery W. R. (1997) Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol. Aging 18, 457–461.
Lovestone S., Hartley C. L., Pearce J., and Anderton B. H. (1996) Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 73(4), 1145–1157.
Lovestone S., Davis D. R., Webster M. T., Kaech S., Brion J. P., Matus A., et al. (1999) Lithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrations. Biol. Psychiatry 45(8), 995–1003.
Mandelkow E. M., Drewes G., Biernat J., Gustke N., Van Lint J., Vandenheede J. R., et al. (1992) Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 314(3), 315–321.
Matsumoto S., Hirano A., and Goto S. (1990) Spinal cord neurofibrillary tangles of Guamanian amyotrophic lateral sclerosis and parkinsonism-dementia complex: an immunohistochemical study. Neurology 40, 975–979.
Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O’Connor M., Trojanowski J. Q., et al. (1994) Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron 13(4), 989–1002.
Mattson M. P. (1990) Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron 4, 105–117.
Mattson M. P. (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77, 1081–1132.
Mattson M. P., Fu W., Waeg G., and Uchida K. (1997) 4-Hydroxynonenal, a product of lipid peroxidation, inhibits dephosphorylation of the microtubule-associated protein tau. Neuroreport 8, 2275–2281.
Mawal-Dewan M., Henley J., Van de Voorde A., Trojanowski J. Q., and Lee V. M.-Y. (1994) The phosphorylation state of tau in the developing rat brain is regulated by phosphoprotein phosphatases. J. Biol. Chem. 269(49), 30,981–30,987.
Mawal-Dewan M., Schmidt M. L., Balin B., Perl D. P., Lee V. M.-Y., and Trojanowski J. Q. (1996) Identification of phosphorylation sites in PHF-TAU from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J. Neuropathol. Exp. Neurol. 55(10), 1051–1059.
McKee A. C., Kosik K. S., and Kowall N. W. (1991) Neuritic pathology and dementia in Alzheimer’s disease. Ann. Neurol. 30(2), 156–165.
McKhann G. M., Albert M. S., Grossman M., Miller B., Dickson D., and Trojanowski J. Q. (2001) Work Group on Frontotemporal Dementia and Pick’s Disease. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch. Neurol. 58(11), 1803–1809.
McQuaid S., Allen I. V., McMahon J., and Kirk J. (1994) Association of measles virus with neurofibrillary tangles in subacute sclerosing panencephalitis: a combined in situ hybridization and immunocytochemical investigation. Neuropathol. Appl. Neurobiol. 20, 103–110.
Mercken M., Fischer I., Kosik K. S., and Nixon R. A. (1995) Three distinct axonal transport rates for tau, tubulin, and other microtubule-associated proteins: evidence for dynamic interactions of tau with microtubules in vivo. J. Neurosci. 15(12), 8259–8267.
Mercken M., Grynspan F., and Nixon R. A. (1995) Differential sensitivity to proteolysis by brain calpain of adult human tau, fetal human tau and PHF-tau. FEBS Lett. 368(1), 10–14.
Merrick S. E., Trojanowski J. Q., and Lee V. M.-Y. (1997) Selective destruction of stable microtubules and axons by inhibitors of protein serine/threonine phosphatases in cultured human neurons. J. Neurosci. 17(15), 5726–5737.
Montine K. S., Reich E., Neely M. D., Sidell K. R., Olson S. J., Markesbery W. R., et al. (1998) Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer disease is associated with APOE genotype. J. Neuropathol. Exp. Neurol. 57, 415–425.
Mori H., Nishimura M., Namba Y., and Oda M. (1994) Corticobasal degeneration: a disease with widespread appearance of abnormal tau and neurofibrillary tangles, and its relation to progressive supranuclear palsy. Acta Neuropathol. (Berl) 88, 113–121.
Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Titani K., et al. (1995) Proline-directed and non-proline-directed phosphorylation of PHF-tau. J. Biol. Chem. 270(2), 823–829.
Munoz-Montano J. R., Moreno F. J., Avila J., and Diaz-Nido J. (1997) Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett. 411(2–3), 183–188.
Murayama S., Mori H., Ihara Y., and Tomonaga M. (1990) Immunocytochemical and ultrastructural studies of Pick’s disease. Ann. Neurol. 27, 394–405.
Murrell J. R., Spillantini M. G., Zolo P., Guazzelli M., Smith M. J., et al. (1999) Tau gene mutation G389R causes a tauopathy with abundant Pick body-like inclusions and axonal deposits. J. Neuropathol. Exp. Neurol. 58(12), 1207–1226.
Nacharaju P., Lewis J., Easson C., Yen S., Hackett J., et al. (1999) Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 447(2–3), 195–199.
Nadelhaft I. (1974) Microtubule densities and total numbers in selected axons of the crayfish abdominal nerve cord. J. Neurocytol. 3(1), 73–86.
Neve R. L., Harris P., Kosik K. S., Kurmit D. M., and Donlon T. A. (1986) Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 387(3), 271–280.
Neve R. L. and Robakis N. K. (1998) Alzheimer’s disease: a re-examination of the amyloid hypothesis. Trends Neurosci. 21(1), 15–19.
Nishimura M., Namba Y., Ikeda K., and Oda M. (1992) Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci. Lett. 143(1–2), 35–38.
Okabe S. and Hirokawa N. (1989) Rapid turnover of microtubule-associated protein MAP2 in the axon revealed by microinjection of biotinylated MAP2 into cultured neurons. Proc. Natl. Acad. Sci. USA 86(11), 4127–4131.
Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., and Tsai L. H. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402(6762), 615–622.
Paulus W. and Selim M. (1990) Corticonigral degeneration with neuronal achromasia and basal neurofibrillary tangles. Acta Neuropathol. (Berl) 81, 89–94.
Perez M., Valpuesta J. M., Medina M., Montejo G., and Avila J. (1996) Polymerization of tau into filaments in the presence of heparin: the minimal sequence required for tau-tau interaction. J. Neurochem. 67(3), 1183–1190.
Perry G., Stwart D., Friedman R., et al. (1987) Filaments of Pick’s bodies contain altered cytoskeletal elements. Am. J. Pathol. 127, 559–568.
Pickering-Brown S., Baker M., Yen S. H., Liu W. K., Hasegawa M., et al. (2000) Pick’s disease is associated with mutations in the tau gene. Ann. Neurol. 48(5), 806–808.
Pollock N. J., Mirra S. S., Binder L. I., Hansen L. A., and Wood J. G. (1986) Filamentous aggregates in Pick’s disease, progressive supranuclear palsy, and Alzheimer’s disease share antigenic determinants with microtubule-associated protein, tau. Lancet 2(8517), 1211.
Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., et al. (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43(6), 815–825.
Probst A., Tolnay M., Langui D., Goedert M., and Spillantini M. G. (1996) Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol. (Berl) 92, 588–596.
Probst A., Götz J., Wiederhold K. H., Tolnay M., Mistl C., et al. (2000) Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 99(5), 469–481.
Reiderer B. and Matus A. (1985) Differential expression of distinct microtubule-associated proteins during brain development. Proc. Natl. Acad. Sci. USA 82(17), 6006–6009.
Reynolds C. H., Utton M. A., Gibb G. M., Yates A., and Anderton B. H. (1997) Stress-activated protein kinase/c-jun N-terminal kinase phosphorylates tau protein. J. Neurochem. 68(4), 1736–1744.
Rizzini C., Goedert M., Hodges J. R., Smith M. J., Jakes R., et al. (2000) Tau gene mutation K257T causes a tauopathy similar to Pick’s disease. J. Neuropathol. Exp. Neurol. 59(11), 990–1001.
Rizzu P., Van Swieten J. C., Joosse M., Hasegawa M., Stevens M., et al. (1999) High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am. J. Hum. Genet. 64(2), 414–421.
Schmidt M. L., Huang R., Martin J. A., et al. (1996) Neurofibrillary tangles in progressive supranuclear palsy contain the same tau epitopes identified in Alzheimer’s disease PHF-tau. J. Neuropathol. Exp. Neurol. 55, 534–539.
Schmidt M. L., Zhukareva V., Perl D. P., Sheridan S. K., Schuck T., Lee V. M.-Y., et al. (2001) Spinal cord neurofibrillary pathology in Alzheimer disease and Guam Parkinsonism-dementia complex. J. Neuropathol. Exp. Neurol. 60(11), 1075–1086.
Schneider A., Biernat J., von Bergen M., Mandelkow E., and Mandelkow E. M. (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38(12), 3549–3558.
Shankar S. K., Yanagihara R., Garruto R. M., Grundke-Iqbal I., Kosik K. S., and Gajdusek D. C. (1989) Immunocytochemical characterization of neurofibrillary tangles in amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Ann. Neurol. 25, 146–151.
Shin R. W., Iwaki T., Kitamoto T., and Tateishi J. (1991) Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and alzheimer’s disease brain tissues. Lab. Investig. 64(5), 693–702.
Snow A. D., Mar H., Nochlin D., Sekiguchi R. T., Kimata K., Koike Y., et al. (1990) Early accumulation of heparin sulfate in neurons and in the beta-amyloid protein-containing lesions of Alzheimer’s disease and Down’s syndrome. Am. J. Pathol. 137(5), 1253–1270.
Sontag E., Nunbhakdi-Craig V., Bloom G. S., and Mumby M. C. (1995) A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J. Cell Biol. 128(6), 1131–1144.
Sontag E., Nunbhakdi-Craig V., Lee G., Bloom G. S., and Mumby M. C. (1996) Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17(6), 1201–1207.
Spillantini M. G., Murrell J. R., Goedert M., Farlow M. R., Klug A., and Ghetti B. (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl. Acad. Sci. USA 95(13), 7737–7741.
Spillantini M. G., Yoshida H., Rizzini C., Lantos P. L., Khan N., et al. (2000) A novel tau mutation (N296N) in familial dementia with swollen achromatic neurons and corticobasal inclusion bodies. Ann. Neurol. 48(6), 939–943.
Spittaels K., Van den Haute C., Van Dorpe J., Bruynseels K., Vandezande K., et al. (1999) Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am. J. Pathol. 155(6), 2153–2165.
Spittaels K., Van den Haute C., Van Dorpe J., Geerts H., Mercken M., Bruynseels K., et al. (2000) Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 275(52), 41,340–41,349.
Stamer K., Vogel R., Thies E., Mandelkow E., and Mandelkow E. M. (2002) Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 156(6), 1051–1063.
Stanford P. M., Halliday G. M., Brooks W. S., Kwok J. B., Storey C. E., et al. (2000) Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain 123(5), 880–893.
Stein-Behrens B., Mattson M. P., Chang I., Yeh M., and Sapolsky R. (1994) Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 14, 5373–5380.
Strittmatter W. J., Saunders A. M., Goedert M., Weisgraber K. H., Dong L. M., Jakes R, et al. (1994) Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc. Natl. Acad. Sci. USA 91(23), 11,183–11,186.
Sturchler-Pierrat C., Abramowski D., Duke M., Wiederhold K. H., Mistl C., et al. (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 94(24), 13,287–13,292.
Suzuki K., Parker C. C., Pentchev P. G., et al. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol. (Berl) 89, 227–238.
Takahashi M., Yasutake K., and Tomizawa K. (1999) Lithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3beta-dependent phosphorylation of juvenile tau in cultured hippocampal neurons. J. Neurochem. 73(5), 2073–2083.
Takei Y., Teng J., Harada A., and Hirokawa N. (2000) Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 150(5), 989–1000.
Tanemura K., Murayama M., Akagi T., Hashikawa T., Tominaga T., Ichikawa M., et al. (2002) Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J. Neurosci. 22(1), 133–141.
Tesseur I., Van Dorpe J., Bruynseels K., Bronfman F., Sciot R., Van Lommel A., et al. (2000) Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am. J. Pathol. 157(5), 1495–1510.
Thal D. R., Arendt T., Waldmann G., Holzer M., Zedlick D., Rub U., et al. (1998) Progression of neurofibrillary changes and PHF-tau in end-stage Alzheimer’s disease is different from plaque and cortical microglial pathology. Neurobiol. Aging 19(6), 517–525.
Thal D. R., Holzer M., Rub U., Waldmann G., Gunzel S., Zedlick D., et al. (2000) Alzheimer-related tau-pathology in the perforant path target zone and in the hippocampal stratum oriens and radiatum correlates with onset and degree of dementia. Exp. Neurol. 163(1), 98–110.
Trinczek B., Biernat J., Baumann K., Mandelkow E. M., and Mandelkow E. (1995) Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol. Biol. Cell 6(12), 1887–1902.
Trojanowski J. Q., Schuck T., Schmidt M. L., and Lee V. M.-Y. (1989) Distribution of tau proteins in the normal human central and peripheral nervous system. J. Histochem. Cytochem. 37(2), 209–215.
Trojanowski J. Q., Schuck T., Schmidt M. L., and Lee V. M.-Y. (1989) Distribution of phosphate-independent MAP2 epitopes revealed with monoclonal antibodies in microwave-denatured human nervous system tissues. J. Neurosci. Methods 29(2), 171–180.
Tsai L. H., Delalle I., Caviness V. S. Jr., Chae T., and Harlow E. (19??) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371(6496), 419–423.
Umahara T., Hirano A., Kato S., et al. (1994) Demonstration of neurofibrillary tangles and neuropil thread-like structures in spinal cord white matter in parkinsonism-dementia complex on Guam and in Guamanian amyotrophic lateral sclerosis. Acta Neuropathol. (Berl) 88, 180–184.
Verbeek M. M., Otte-Holler I., van den Born J., van den Heuvel L. P., David G., Wesseling P., et al. (1999) Agrin is major heparin sulfate proteoglycan accumulating in Alzheimer’s disease brain. Am. J. Pathol. 155(6), 2115–2125.
Viereck C., Tucker R. P., Binder L. I., and Matus A. (1990) Phylogenetic conservation of brain microtubule-associated proteins MAP2 and tau. Neuroscience 26(3), 893–904.
Vogelsberg-Ragaglia V., Bruce J., Richter-Lansberg C., Zhang B., Hong M., et al. (2000) Distinct FTDP-17 missense mutations in tau produce tau aggregates and other phathological phenotypes in transfected CHO cells. Mol. Biol. Cell 11(12), 4093–4104.
Vogelsberg-Ragaglia V., Schuck T., Trojanowski J. Q., and Lee V. M.-Y. (2001) PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp. Neurol. 168(2), 402–412.
Wakabayashi K., Oyanagi K., Makifuchi T., et al. (1994) Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. (Berl) 87, 545–553.
Watanabe A., Hasegawa M., Suzuki M., Takio K., Morishima-Kawashima M., Titani K., et al. (1993) In vivo phosphorylation sites in fetal and adult rat tau. J. Biol. Chem. 268(34), 25,712–25,717.
Weingarten M. D., Lockwood A. H., Hwo S. Y., and Kirschner M. W. (1975) A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 72(5), 1858–1862.
Wilhelmsen K. C., Lynch T., Pavlou E., et al. (1994) Localization of disnhibition-dementia-parkinsonism-amyotrophy complex to 17q21-22. Am. J. Hum. Gen. 55, 1159–1165.
Wilson D. M. and Binder L. I. (1997) Free fatty acids simulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer’s disease. Am. J. Pathol. 150(6), 2181–2195.
Wischik C. M., Novak M., Thogersen H. C., Edwards P. C., Runswick M. J., Jakes R., et al. (1988) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. USA 85(12), 4506–4510.
Woodgett J. R. (1990) Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 9(8), 2431–2438.
Yamada T., McGeer P. L., and McGeer E. G. (1992) Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci. Lett. 135(1), 99–102.
Yamazaki M., Nakano I., Imazu O., Kaieda R., and Terashi A. (1994) Astrocytic straight tubules in the brain of a patient with Pick’s disease. Acta Neuropathol. (Berl) 88(6), 587–591.
Yasuda M., Takamatsu J., D’Souza I., Crowther R. A., Kawamata T., et al. (2000) A novel mutation at posititon +12 in the intron following exon 10 of the tau gene in familial frontotemporal dementia (FTD-Kumamoto). Ann. Neurol. 47(4), 422–429.
Yoshida H. and Ihara Y. (1993) Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament-tau. J. Neurochem. 61(3), 1183–1186.
Zheng-Fischhofer Q., Biernat J., Mandelkow E. M., Illenberger S., Godemann R., and Mandelkow E. (1998) Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur. J. Biochem. 252(3), 542–552.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higuchi, M., Lee, V.MY. & Trojanowski, J.Q. Tau and axonopathy in neurodegenerative disorders. Neuromol Med 2, 131–150 (2002). https://doi.org/10.1385/NMM:2:2:131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/NMM:2:2:131