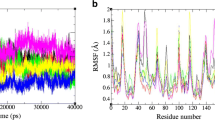
Abstract
Africa accounts for the majority of HIV-1 infections worldwide caused mainly by the A and C viral subtypes rather than B subtype, which prevails in the United States and Western Europe. In Brazil, B subtype is the major subtype, but F, C, and A also circulate. These non-B subtypes present polymorphisms, and some of them occur at sites that have been associated with drug resistance, including the HIV-1 protease (PR), one important drug target. Here, we report a Molecular Dynamics study of the B and non-B PR complexed with the inhibitor ritonavir to delineate the behavior of each subtype. We compare root mean squared deviation, binding free energy by linear interaction energy approach, hydrogen bonds, and intermolecular contact surface area between inhibitor and PR. From our results, we can provide a basis to understand the molecular mechanism of drug resistance in non-B subtypes. In this sense, we found a decrease of approx 4 kcal/mol in ΔG of binding between B and non-B subtypes. This corresponds to the loss of one hydrogen bond, which is in agreement with our H-bond analysis. Previous experimental affinity studies reported analogous results with inhibition constant values for non-B PR.
Similar content being viewed by others
References
Hu W. S. and Temin, H. M. (1990) Retroviral recombination and reverse transcription. Science 250, 1227–1233.
Preston, B. D., Poiesz, B. J. and Loeb, L. A. (1988) Fidelity of HIV-1 reverse transcriptase. Science 242, 1168–1171.
Ho, D. D., Neumann, A. U., Perelson, A. S., Chen, W., Leonard, J. M. and Markowitz, M. (1995) Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373, 123–126.
Simon, F., Mauclere, P., Roques, P., et al. (1998) Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat. Med. 4, 1032–1037.
Kantor, R. and Katzenstein, D. (2003) Polymorphism in HIV-1 non-subtype B protease and reverse transcriptase and its potential impact on drug susceptibility and drug resistance evolution. AIDS Rev. 5, 25–35.
Wainberg, M. A. (2004) HIV-1 subtype distribution and the problem of drug resistance. AIDS 18 (Suppl.) 3), S63-S68.
UNAIDS (2004) AIDS epidemic update: 2004. UNAIDS/WHO, Geneva, Switzerland.
Osmanov, S., Pattou, C., Walker, N., Schwardlander, B., Esparza, J. and Charact, W.-U.N.H.I. (2002) Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J. Acquir. Immun. Defic. Syndr. 29, 184–190.
Soares, M. A., Brindeiro, R. M. and Tanuri, A. (2004) Primary HIV-1 drug resistance in Brazil. AIDS 18 (Suppl.) 3, S9-S13.
Prabu-Jeyabalan, M., Nalivaika, E. and Schiffer, C. A. (2000) How does a symmetric dimer recognize an asymmetric substrate? A substrate complex of HIV-1 protease. J. Mol. Biol. 301, 1207–1220.
Freedberg, D. I., Ishima, R., Jacob, J., et al. (2002) Rapid structural fluctuations of the free HIV protease flaps in solution: relationship to crystal structures and comparison with predictions of dynamics calculations. Protein Sci. 11, 221–232.
Sanches, M., Martins, N. H., Calazans, A., et al. (2004) Crystallization of a non-B and a B mutant HIV protease. Acta Crystallogr. D. Biol. Crystallogr. 60, 1625–1627.
Wlodawer, A. and Vondrasek, J. (1998) Inhibitors of HIV-1 protease: a major success of structure-assisted drug design. Annu. Rev. Biophys. Biomol. Struct. 27, 249–284.
Berman, H. M., Westbrook, J., Feng, Z. et al. (2000) The Protein Data Bank. Nucleic Acids Res. 28, 235–242.
Velazquez-Campoy, A., Vega, S., Fleming, et al. (2003) Protease inhibition in African subtypes of HIV-1. AIDS Rev. 5, 165–171.
Velazquez-Campoy, A., Todd, M. J., Vega, S. and Freire, E. (2001) Catalytic efficiency and vitality of HIV-1 proteases from African viral subtypes. Proc. Natl. Acad. Sci. USA 98, 6062–6067.
Vicente, A. C., Agwale, S. M., Otsuki, K., et al. (2001) Genetic variability of HIV-1 protease from Nigeria and correlation with protease inhibitors drug resistance. Virus Genes 22, 181–186.
Soares, M. A., De Oliveira, T., Brindeiro, R. M., et al. (2003) A specific subtype C of human immunodeficiency virus type 1 circulates in Brazil. AIDS 17, 11–21.
Caride, E., Hertogs, K., Larder, B., et al. (2001) Genotypic and phenotypic evidence of different drugresistance mutation patterns between B and non-B subtype isolates of human immunodeficiency virus type 1 found in Brazilian patients failing HAART. Virus Genes 23, 193–202.
Guex, N. and Peitsch, M. C. (1997) SWISS-MODEL and the Swiss-Pdb Viewer; an environment for comparative protein modeling. Electrophoresis 18, 2714–2723.
Kempf D. J., Marsh, K. C., Denissen, J. F., et al. (1995) ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc. Natl. Acad. Sci. USA 92, 2484–2488.
Laskowski, R. A., Rullmann, J. A., MacArthur M. W., Kaptein, R., and Thornton, J. M. (1996) AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486.
van Gunsteren, W. F., Billeter, S. R., Eising, A. A., et al. (1996) Biomolecular Simulation: The GROMOS96 Manual and User Guide. vdt Hochschulverlag AG an der ETH Zürich and BIOMOS b.v., Zürich Groningen.
van Aalten, D. M., Bywater, R., Findlay, J. B., Hendlich, M., Hooft, R. W. and Vriend, G. (1996) PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J. Comput. Aided Mol. Des, 10, 255–262.
van Gunsteren, W. F. and Berendsen, H.J.C. (1987) Groningen Molecular Simulation (GROMOS) Library Manual. BIOMOS b.v., Groningen.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. (1995) GAUSSIAN94, Revision B.1 Gaussian, Inc., Pittsburgh, PA.
van der Spoel, D., van Buuren, A. R., Apol,, E., et al. (2001) Gromacs User’s Manual version 3.0, Groningen.
Berendsen, H.J.C., van der Spoel, D., and van Drunen, R. (1995) GROMACS: A message-passing parallel molecular dynamics implementation. Comp. Phys. Commun. 91, 43–56.
Humphrey, W., Dalke, A. and Schulten, K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38,27–38.
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. V., and Hermans, J. (1981) Interaction models for water in relation to protein hydration, in Intermolecular Forces (Pullman, B., ed.), Reidel, Dordrecht, The Netherlands, pp. 331–342.
Hess, B., Bekker, H., Berendsen, H. J. C. and Fraaije, J. G. E. M. (1997) LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472.
Miyamoto, S. and Kollman, P. A. (1992) Settle-an analytical version of the shake and rattle algorithm for rigid water models. J. Comput. Chem. 13, 952–962.
Berendsen, H. J. C., Postma, J. P. M., Vangunsteren, W. F., Dinola, A., and Haak, J. R. (1984) Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690.
Schreiber, H. and Steinhauser, O. (1992) Taming cut-off induced artifacts in molecular dynamics studies of solvated polypeptides. The reaction field method. J. Mol. Biol. 228, 909–923.
Smith, P. E. and Vangunsteren, W. F. (1994) Consistent dielectric-properties of the simple point-charge and extended simple point-charge water models at 277 and 300 K. J. Chem. Phys. 100, 3169–3174.
Hyland, L. J. Tomaszek, T. A., Jr., Roberts, G. D., et al. (1991) Human immunodeficiency virus-1 protease 1. Initial velocity studies and kinetic characterization of reaction intermediates by 18O isotope exchange. Biochemistry 30, 8441–8453.
Hyland, L. J., Tomaszek, T. A., Jr. and Meek, T. D. (1991) Human immunodeficiency virus-1 protease. 2. Use of pH rate studies and solvent kinetic isotope effects to elucidate details of chemical mechanism. Biochemistry 30, 8454–8463.
Okimoto, N., Tsukui, T., Hata, M., Hoshino, T. and Tsuda, M. (2000) Molecular dynamics study of HIV-1 proteasesubstrate complex: roles of the water molecules at the loop structures of the active site. J. Am. Chem. Soc. 122, 5613–5622.
Aqvist, J., Medina, C. and Samuelsson, J. E. (1994) A new method for predicting binding affinity in computer-aided drug design. Protein Eng. 7, 385–391
Hulten, J., Bonham, N. M., Nillroth, U., et al. (1997) Cyclic HIV-1 protease inhibitors derived from mannitol: synthesis, inhibitory potencies, and computational predictions of binding affinities. J. Med. Chem. 40, 885–897.
Wang, W., Wang, J. and Kollman, P. A. (1999) What determines the van der Waals coefficient beta in the LIE (linear interaction energy) method to estimate binding free energies using molecular dynamics simulations? Proteins 34, 395–402.
Aqvist, J., Luzhkov, V. B. and Brandsdal, B. O. (2002) Ligand binding affinities from MD simulations. Accounts Chem. Res. 35, 358–365.
Connolly, M. L. (1983) Solvent-accessible surfaces of proteins and nucleic-acids. Science 221, 709–713.
Koradi, R., Billeter, M. and Wuthrich, K. (1996) MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55, 29–32.
Wang, W. and Kollman, P. A. (2001) Computational study of protein specificity: the molecular basis of HIV-1 protease drug resistance. Proc. Natl. Acad. Sci. USA. 98, 14,937–14,942.
Brandsdal, B. O., Osterberg, F., Almlof, M., Feierberg, I., Luzhkov, V. B. and Aqvist, J. (2003) Free energy calculations and ligand binding. Adv. Protein Chem. 66, 123–158.
Garrett, R. and Grisham, C. M. (1995) Biochemistry, Saunders College Pub, Fort Worth, TX.
Ala, P. J., Huston, E. E., Klabe, R. M., et al. (1997) Molecular basis of HIV-1 protease drug resistance: structural analysis of mutant proteases complexed with cyclic urea inhibitors. Biochemistry 36, 1573–1580.
Deeks, S. G., Smith, M., Holodniy, M. and Kahn, J. O. (1997) HIV-1 protease inhibitors-a review for clinicians. J. Am. Med. Assoc. 277, 145–153.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Batista, P.R., Wilter, A., Durham, E.H.A.B. et al. Molecular dynamics simulations applied to the study of subtypes of HIV-1 protease common to Brazil, Africa, and Asia. Cell Biochem Biophys 44, 395–404 (2006). https://doi.org/10.1385/CBB:44:3:395
Issue Date:
DOI: https://doi.org/10.1385/CBB:44:3:395