Abstract
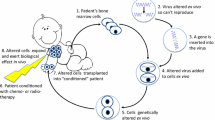
This review describes the main steps which led to carrying out the gene ther-apy clinical trial for Xlinked severe combined immune deficiency (SCID-X1) patients, from the preparation of the retroviral vector up to the design of GMP conditions to transduce ex vivo the CD34+cells of patients. This gene therapy protocol is currently being applied and the encouraging preliminary results published (1). The success of this protocol could be mainly attributed to the physiopathology of the SCID-X1 disease (for review, see ref. 2), which is a good model for a gene therapy application. However, progress in the technology of gene transfer into hematopoietic progenitor cells (HPCs) may also have contributed to this success. The most important improvement concerns the use of fibronectin, which increases the transduction efficiency by colocalization of the retroviral particles and the HPC. The other improvement is related to the use of the early-acting cytokines such as stem cell factor (SCF), Flt3 ligand, and megakaryocyte growth and differentiation factor (M-GDF), which promote immature CD34+CD38low cycle induction, making them permissive for transgene integration with minimal loss of their lymphoid potential (3)
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Cavazzana-Calvo, M., Hacein-Bey, S., de Saint Basile, G., Gross, F., Y von, E., Nusbaum, P., et al. (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672.
Fischer, A. (2000) Severe combined immunodeficiencies (SCID). Clin. Exp. Immunol. 122143–149.
Hacein-Bey, S., Gross, F., Nusbaum, P., Hue, C., Hamel, Y., Fischer, A., et al. (2001) Optimization of retroviral gene transfer protocol to maintain the lymphoid potential of progenitor cells. Hum. Gene Ther. 12, 291–301.
Danos, O. and Mulligan, R. C. (1988) Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci.USA 85, 6460–6464.
Riviere, I., Brose, K., and Mulligan, R. C. (1995) Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl. Acad. Sci. USA 92, 6733–6737.
Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning, A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Moritz, T., Dutt, P., Xiao, X., Carstanjen, D., Vik, T., Hanenberg, H., et al. (1996) Fibronectin improves transduction of reconstituting hematopoietic stem cells by retroviral vectors: evidence of direct viral binding to chymotryptic carboxy-terminal fragments. Blood 88, 855–862.
Hanenberg, H., Xiao, X. L., Dilloo, D., Hashino. K., Kato, I., and Williams, D. A. (1996) Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat. Med. 2, 876–882.
Hacein-Bey, S., Basile, G. D., Lemerle, J., Fischer, A., and Cavazzana-Calvo, M. (1998) Gamma c gene transfer in the presence of stem cell factor, FLT-3L, interleukin-7 (IL-7), IL-1, and IL-15 cytokines restores T-cell differentiation from gammac(-) X-linked severe combined immunodeficiency hematopoietic progenitor cells in murine fetal thymic organ cultures. Blood 92, 4090–4097.
Cavazzana-Calvo, M., Hacein-Bey, S., de Saint Basile, G., De Coene, C., Selz, F., Le Deist, F., et al. (1996) Role of interleukin-2 (IL-2), IL-7, and IL-15 in natural killer cell differentiation from cord blood hematopoietic progenitor cells and from gamma c transduced severe combined immunodeficiency X1 bone marrow cells. Blood 88, 3901–3909.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2003 Humana Press Inc.
About this protocol
Cite this protocol
Hacein-Bey-Abina, S., Saint Basile, G.d., Cavazzana-Calvo, M. (2003). Gene Therapy of X-Linked Severe Combined Immunodeficiency. In: Körholz, D., Kiess, W. (eds) Cytokines and Colony Stimulating Factors. Methods in Molecular Biology, vol 215. Humana, Totowa, NJ. https://doi.org/10.1385/1-59259-345-3:247
Download citation
DOI: https://doi.org/10.1385/1-59259-345-3:247
Publisher Name: Humana, Totowa, NJ
Print ISBN: 978-1-58829-035-9
Online ISBN: 978-1-59259-345-3
eBook Packages: Springer Protocols