-
PDF
- Split View
-
Views
-
Cite
Cite
Dihua Shangguan, Zehui Charles Cao, Ying Li, Weihong Tan, Aptamers Evolved from Cultured Cancer Cells Reveal Molecular Differences of Cancer Cells in Patient Samples, Clinical Chemistry, Volume 53, Issue 6, 1 June 2007, Pages 1153–1155, https://doi.org/10.1373/clinchem.2006.083246
Close - Share Icon Share
Abstract
Background: Molecular-level differentiation of neoplastic cells is essential for accurate and early diagnosis, but effective molecular probes for molecular analysis and profiling of neoplastic cells are not yet available. We recently developed a cell-based SELEX (systematic evolution of ligands by exponential enrichment) strategy to generate aptamers (designer DNA/RNA probes) as molecular probes to recognize neoplastic cells.
Methods: We tested 6 cell-SELEX–generated aptamers with equilibrium dissociation constants in the nanomolar to subnanomolar range: sgd5, selected from Toledo cells, a human diffuse large-cell lymphoma cell line (B-cell), and sgc8, sgc3, sgc4, sgd2, and sgd3 from CCRF-CEM cells, a human precursor T cell acute lymphoblastic leukemia (T-ALL) cell line. Aptamers were labeled with fluorescein isothiocyanate fluorophores and then used to recognize, by flow cytometric analysis, neoplastic cells in cultured hematopoietic cell lines and clinical samples.
Results: Aptamer sgd5 recognized only its target cells. Aptamers sgc3, sgd2, sgd3, sgc4, and sgc8, selected from a T-cell leukemia cell line, identified all of the cultured T-cell leukemia cell lines with relatively high fluorescence intensity. Aptamers sgc8, sgc3, and sgd3 showed good selectivity toward T-ALL cells and almost no binding to normal hematopoietic cells or lymphoma and myeloma cells. Selected aptamers also detected targets on the cell membranes of neoplastic cells in patient samples.
Conclusions: Aptamers selected against cultured neoplastic cells can effectively be used as molecular probes for recognition of neoplastic cells in patient samples. Cell-based aptamer selection can be used to generate aptamer probes to obtain molecular signatures of neoplastic cells in patient samples.
Complex genetic and proteomic alterations reveal the molecular heterogeneity within individual cancer diagnostic categories. Identification and understanding of the molecular basis of diseased cells should provide the most reliable approach toward effective diagnosis and treatments. Given the complexity and diversity of cancers, even within similar categories, multiple cancer-specific molecular probes are needed to delineate unique fingerprints of tumor cells. Molecular-level differences exist between any 2 given types of cells, even 2 individual cases of a same tumor type, but for most diseases no reliable molecular probes are specific enough to recognize these subtle molecular differences (1). A critically important task for molecular medicine is to identify these differences and then use them to further characterize and understand the molecular basis of diseases. Despite the variety of clinical variables used to classify human malignancies, most neoplastic diseases cannot be defined or classified according to abnormal molecular disease processes because of the lack of molecular probes that can be used define these processes. Thus, patients receiving similar diagnoses can have markedly different clinical courses and responses to treatments (2).
Currently, the diagnosis of leukemia is commonly based on morphologic evaluation supplemented by immunophenotype analysis by flow cytometry with monoclonal antibodies of CD antigens (3). These antigens are usually expressed on both normal and neoplastic cells, however, and thus cannot accurately reflect the molecular features of the cancer cells. Although many antibodies are available for phenotyping leukemia, they were not developed to enable comprehensive recognition of molecular features of specific disease cells but were individually developed at different times for various purposes. Systematic production of a panel of antibodies for molecular differentiation of cancer cells would be very difficult because of the technical difficulties involved in systematic development of antibodies for unknown surface biomarkers. Novel approaches are therefore needed to systematically generate panels of new probes that recognize molecular signatures of cancers.
We previously developed panels of DNA aptamers, single-stranded oligonucleotides, directly from live tumor cells with a process called SELEX (systematic evolution of ligands by exponential enrichment) (4)(5)(6). These aptamers recognized surface targets of cancer cells with high affinity and specificity. Selected aptamers can bind to target molecules by folding into well-defined 3-dimensional structures (5)(6)(7). Unlike antibodies, aptamers, once the sequence is known, can be synthesized reproducibly by a DNA synthesizer at a very low cost (8)(9)(10)(11)(12)(13). In addition, aptamers have low molecular weight, fast tissue penetration, and low toxicity. They can be specifically labeled with radioscopic, fluorescent, or other reporters for molecular recognition. Moreover, aptamers are stable during long-term storage, can be transported at ambient temperature, and sustain reversible denaturation. Despite their advantages and unique properties as molecular probes, however, aptamers have been used sparingly for medical applications because of the limited number of available aptamers that have medical relevance. Acquisition of aptamers directly from diseased cells is expected to link aptamers more closely to real medical problems and to greatly reduce the time gap between laboratory research and clinical applications.
We report a group of new aptamers selected directly from cancer cells (4) for the recognition of molecular differences among leukemia patient samples. These aptamers have high affinity and specificity for surface targets of neoplastic cells in clinical samples, and bind with clinical samples to form distinct recognition patterns. Thus, these aptamers may be useful for both disease diagnosis and efficient personalized therapy for individual patients.
Aptamers are usually selected for single target molecules. In contrast, cell-SELEX elects aptamers by use of complex whole cells as targets (4). A counterselection strategy is used to isolate aptamer sequences that interact only with the target cells and not with the control cells. Through this process, a group of cell-specific aptamers can be selected in a relatively short period (4–8 weeks) even if it is not known which target molecules are present on the cell surface and which membrane molecules might play the most important role in cancer development. This feature is the most important difference between cell-SELEX and other current methods of molecular probe development, and enables cell-SELEX to generate multiple molecular probes to recognize biomarkers in their native states, producing molecular signatures of diseases.
We used the cell-SELEX to obtain many aptamers with high affinity and specificity to surface molecules on target cancer cells. We then chose 6 aptamers with equilibrium dissociation constants in the nanomolar to subnanomolar range: sgd5—selected from Toledo cells, a human diffuse large-cell lymphoma cell line (B-cell)—and sgc3, sgd2, sgd3, sgc4, and sgc8 from CCRF-CEM cells, a human T precursor T cell acute lymphoblastic leukemia (T-ALL) cell line.
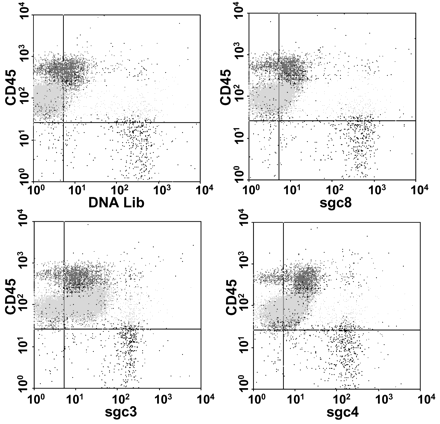
The 6 selected aptamers were first conjugated with fluorescein isothiocyanate (FITC) for recognition of different kinds of cells. We then used flow cytometric analysis to monitor the binding of aptamers to cells from 4 T-cell leukemia cell lines; 8 B-cell lymphoma, leukemia, or myeloma cell lines; and normal human bone marrow aspirates (see the methods and materials in the Data Supplement that accompanies the online version of this Technical Brief at www.clinchem.org/content/vol53/issue6). Subpopulations of bone marrow cells were identified in the flow cytometric analysis by their side-scatter properties and the expression of CD3, CD7, CD10, CD19, and CD45. The following cell types were identified: mature B cells, immature B cells, CD3(+) T cells, monocytes, granulocytes, and nucleated erythrocytes. The dot plot of a typical flow cytometry analysis is shown in Fig. 11 . The FITC-labeled DNA library was measured as the background binding, and a threshold was determined based on the background fluorescence. Cells recognized by aptamers were expressed as percentage of cells with fluorescence higher than the background threshold. The results (see Table 1 in the online Data Supplement) showed that aptamer sgd5 recognized only its target cells. All of the cultured T-cell leukemia cell lines were identified with relatively high fluorescence intensity by aptamers sgc3, sgd2, sgd3, sgc4, and sgc8, which was expected because they were selected from a T-cell leukemia cell line. Aptamers sgc8, sgc3, and sgd3 showed good selectivity toward T-ALL cells and almost no binding to normal hematopoietic cells in the human bone marrow samples or lymphoma and myeloma cells. Further inspection showed that aptamers sgc4 and sgd2 recognized many different cell samples, including some normal bone marrow cells, indicating the presence of common binding entities on these cells. Combination of selected aptamers produced distinct patterns for different tumor cells, suggesting that aptamers may be used to define molecular signatures of tumors.
Selected aptamers also detected targets on the cell membranes of neoplastic cells in patient samples, including T-ALL, B-cell ALL, acute myeloid leukemia (AML), and lymphomas (Table 11 ). All lymphoma samples with mature T or B cells showed no or very low binding (see Table 1 in the online Data Supplement), indicating that recognition was not due to nonspecific interactions, in agreement with the fact that most of the aptamers were selected against a cultured precursor T-ALL cell line. As expected, the aptamers showed more binding with cells from T-ALL patients than with other cell samples. Aptamer binding patterns corresponded well with general categories of acute leukemia predefined by antibody immunophenotyping.
Despite the results showing that aptamers can selectively recognize cultured T-ALL cells, demonstrating the specificity of selected aptamers (Table 11 ), individual cases of clinical specimens may have quite different patterns even in the same disease category. Although the explanation for these differences remains unknown, they precisely reflect the complex nature of the disease. In addition to general categorization of the leukemia suggested by available antibodies, our aptamer analyses provide direct evidence for the subtle molecular differences among the same type of cancers. It is well known that responses to specific treatments may differ among diseases of the same category (1)(2), but confirmation of dissimilarities at the molecular level has been hindered by technical difficulties and lack of specific molecular probes. The cell-SELEX method (4) may provide a simple, fast, and low-cost way to generate panels of molecular probes and reveal subtle differences even before specific disease biomarkers are known.
In conclusion, with aptamers directly evolved from T-ALL cells we were able to identify leukemia cells in patient samples and detect subtle molecular differences among individual samples from leukemia patients in the same category. Our results demonstrate that cell-based aptamer selection can be a valuable approach for generating aptamer probes to obtain molecular signatures of individual patient samples. Although the molecular profile or signature may not necessarily indicate the detailed molecular mechanism of a disease, it may be the first step toward understanding the molecular basis of a disease. Because of features such as chemical-synthesis–based production, low molecular weight, easy modification, and long-term stability after modification, aptamers selected from cancer cells may be effective molecular probes for cancer diagnosis.
Molecular recognition of T-ALL cells in patient bone marrow aspirates with FITC-labeled sgc8’sgc3, sgc4, and PerCP-labeled anti-CD45 antibody.
The background was measured by using FITC-labeled unselected library. The light gray dots represent T-ALL cells.
Aptamer profiling of cancer cells in patients’samples.1
| . | sgc8 . | sgc3 . | sgc4 . | sgd2 . | sgd3 . | sgd5 . |
|---|---|---|---|---|---|---|
| T ALL 1 | ++ | +++ | +++ | +++ | +++ | ND |
| T ALL 2 | ++ | + | +++ | ++ | + | 0 |
| T ALL 3 | + | + | ++++ | +++ | + | 0 |
| T ALL 4 | + | + | ++ | +++ | + | 0 |
| T ALL 5 | + | + | ++ | + | + | 0 |
| T ALL 6 | 0 | 0 | + | + | 0 | 0 |
| T ALL 7 | 0 | 0 | ++ | ++ | 0 | 0 |
| TALL 8 | + | + | ++ | ++ | + | 0 |
| TALL 9 | + | 0 | + | + | 0 | 0 |
| TALL10 | 0 | + | + | 0 | + | 0 |
| B ALL 1 | 0 | 0 | ++ | ++ | 0 | 0 |
| B ALL 2 | 0 | 0 | ++ | ++ | 0 | + |
| B ALL 3 | ++ | 0 | ++ | ++ | 0 | + |
| B-ALL 4 | 0 | 0 | + | + | 0 | 0 |
| AML 1 | + | + | ++ | + | 0 | 0 |
| AML 2 | + | 0 | ++ | + | 0 | 0 |
| AML 3 | + | 0 | + | + | 0 | 0 |
| AML 4 | 0 | 0 | ++++ | ++++ | 0 | 0 |
| AML 5 | 0 | 0 | + | 0 | 0 | 0 |
| AML 6 | + | 0 | 0 | 0 | 0 | 0 |
| AML 7 | + | 0 | 0 | 0 | 0 | 0 |
| AML 8 | + | 0 | +++ | +++ | 0 | 0 |
| . | sgc8 . | sgc3 . | sgc4 . | sgd2 . | sgd3 . | sgd5 . |
|---|---|---|---|---|---|---|
| T ALL 1 | ++ | +++ | +++ | +++ | +++ | ND |
| T ALL 2 | ++ | + | +++ | ++ | + | 0 |
| T ALL 3 | + | + | ++++ | +++ | + | 0 |
| T ALL 4 | + | + | ++ | +++ | + | 0 |
| T ALL 5 | + | + | ++ | + | + | 0 |
| T ALL 6 | 0 | 0 | + | + | 0 | 0 |
| T ALL 7 | 0 | 0 | ++ | ++ | 0 | 0 |
| TALL 8 | + | + | ++ | ++ | + | 0 |
| TALL 9 | + | 0 | + | + | 0 | 0 |
| TALL10 | 0 | + | + | 0 | + | 0 |
| B ALL 1 | 0 | 0 | ++ | ++ | 0 | 0 |
| B ALL 2 | 0 | 0 | ++ | ++ | 0 | + |
| B ALL 3 | ++ | 0 | ++ | ++ | 0 | + |
| B-ALL 4 | 0 | 0 | + | + | 0 | 0 |
| AML 1 | + | + | ++ | + | 0 | 0 |
| AML 2 | + | 0 | ++ | + | 0 | 0 |
| AML 3 | + | 0 | + | + | 0 | 0 |
| AML 4 | 0 | 0 | ++++ | ++++ | 0 | 0 |
| AML 5 | 0 | 0 | + | 0 | 0 | 0 |
| AML 6 | + | 0 | 0 | 0 | 0 | 0 |
| AML 7 | + | 0 | 0 | 0 | 0 | 0 |
| AML 8 | + | 0 | +++ | +++ | 0 | 0 |
In the flow cytometry analysis, a threshold based on fluorescence intensity of FITC was chosen so that 99% percent of cells incubated with the FITC-labeled unselected DNA library would have fluorescence intensity below it. When FITC-labeled aptamer was allowed to interact with the cells, the percentage of the cells with fluorescence above the set threshold was used to evaluate the binding capacity of the aptamer to the cells. 0, <10%; +, 10%—35%; ++,35%—60%; +++, 60%—85%; ++++, >85%.
Aptamer profiling of cancer cells in patients’samples.1
| . | sgc8 . | sgc3 . | sgc4 . | sgd2 . | sgd3 . | sgd5 . |
|---|---|---|---|---|---|---|
| T ALL 1 | ++ | +++ | +++ | +++ | +++ | ND |
| T ALL 2 | ++ | + | +++ | ++ | + | 0 |
| T ALL 3 | + | + | ++++ | +++ | + | 0 |
| T ALL 4 | + | + | ++ | +++ | + | 0 |
| T ALL 5 | + | + | ++ | + | + | 0 |
| T ALL 6 | 0 | 0 | + | + | 0 | 0 |
| T ALL 7 | 0 | 0 | ++ | ++ | 0 | 0 |
| TALL 8 | + | + | ++ | ++ | + | 0 |
| TALL 9 | + | 0 | + | + | 0 | 0 |
| TALL10 | 0 | + | + | 0 | + | 0 |
| B ALL 1 | 0 | 0 | ++ | ++ | 0 | 0 |
| B ALL 2 | 0 | 0 | ++ | ++ | 0 | + |
| B ALL 3 | ++ | 0 | ++ | ++ | 0 | + |
| B-ALL 4 | 0 | 0 | + | + | 0 | 0 |
| AML 1 | + | + | ++ | + | 0 | 0 |
| AML 2 | + | 0 | ++ | + | 0 | 0 |
| AML 3 | + | 0 | + | + | 0 | 0 |
| AML 4 | 0 | 0 | ++++ | ++++ | 0 | 0 |
| AML 5 | 0 | 0 | + | 0 | 0 | 0 |
| AML 6 | + | 0 | 0 | 0 | 0 | 0 |
| AML 7 | + | 0 | 0 | 0 | 0 | 0 |
| AML 8 | + | 0 | +++ | +++ | 0 | 0 |
| . | sgc8 . | sgc3 . | sgc4 . | sgd2 . | sgd3 . | sgd5 . |
|---|---|---|---|---|---|---|
| T ALL 1 | ++ | +++ | +++ | +++ | +++ | ND |
| T ALL 2 | ++ | + | +++ | ++ | + | 0 |
| T ALL 3 | + | + | ++++ | +++ | + | 0 |
| T ALL 4 | + | + | ++ | +++ | + | 0 |
| T ALL 5 | + | + | ++ | + | + | 0 |
| T ALL 6 | 0 | 0 | + | + | 0 | 0 |
| T ALL 7 | 0 | 0 | ++ | ++ | 0 | 0 |
| TALL 8 | + | + | ++ | ++ | + | 0 |
| TALL 9 | + | 0 | + | + | 0 | 0 |
| TALL10 | 0 | + | + | 0 | + | 0 |
| B ALL 1 | 0 | 0 | ++ | ++ | 0 | 0 |
| B ALL 2 | 0 | 0 | ++ | ++ | 0 | + |
| B ALL 3 | ++ | 0 | ++ | ++ | 0 | + |
| B-ALL 4 | 0 | 0 | + | + | 0 | 0 |
| AML 1 | + | + | ++ | + | 0 | 0 |
| AML 2 | + | 0 | ++ | + | 0 | 0 |
| AML 3 | + | 0 | + | + | 0 | 0 |
| AML 4 | 0 | 0 | ++++ | ++++ | 0 | 0 |
| AML 5 | 0 | 0 | + | 0 | 0 | 0 |
| AML 6 | + | 0 | 0 | 0 | 0 | 0 |
| AML 7 | + | 0 | 0 | 0 | 0 | 0 |
| AML 8 | + | 0 | +++ | +++ | 0 | 0 |
In the flow cytometry analysis, a threshold based on fluorescence intensity of FITC was chosen so that 99% percent of cells incubated with the FITC-labeled unselected DNA library would have fluorescence intensity below it. When FITC-labeled aptamer was allowed to interact with the cells, the percentage of the cells with fluorescence above the set threshold was used to evaluate the binding capacity of the aptamer to the cells. 0, <10%; +, 10%—35%; ++,35%—60%; +++, 60%—85%; ++++, >85%.
Grant/funding support: This work was supported by National Institutes of Health grants and a National Science Foundation Nanotechnology Interdisciplinary Research Team grant.
Financial disclosures: None declared.
Espina V, Geho D, Mehta AI, Petricoin EF, III, Liotta LA, Rosenblatt KP. Pathology of the future: molecular profiling for targeted therapy.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling.
Belov L, dela Vega O, dos Remedios CG, Mulligan SP, Christopherson R. Immunophenotyping of leukemias using a cluster of differentiation antibody microarray.
Shangguan D, Li Y, Tang Z, Cao Z, Chen H, Mallikratchy P, et al. Aptamers evolved from live cells as effective molecular probes for cancer study.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands.
Breaker RR. Natural and engineered nucleic acids as tools to explore biology.
Yang CJ, Jockusch S, Vicens M, Turro N, Tan W. Light-switching excimer probes for rapid protein monitoring in complex biological fluids.
Nutiu R, Li Y. In vitro selection of structure-switching signaling aptamers.
Liu JW, Lu Y. Fast colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticles.
Blank M, Weinschenk T, Priemer M, Schluesener H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels.
Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment.