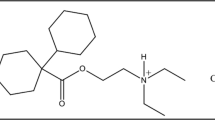
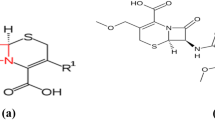
Abstract
A liquid chromatographic method has been applied for the analysis of two antibiotics widely used in the veterinary field, oxytetracycline dihydrate and chlortetracycline hydrochloride in premixes for medicated feeding stuffs for veterinary use. In particular, the validated method was employed to study the releasing profile of each drug from two formulations, a commercially available and a new formulation, having different excipient composition. The dissolution profiles obtained from the chromatographic analysis allowed to verify the in vitro bio-equivalence of the commercial and the new formulations for oxytetracycline and chlortetracycline.




Similar content being viewed by others
References
Costa P, Lobo J (2001) Eur J Pharm Sci 13:123–133. doi:10.1016/S0928-0987(01)00095-1
Liang Y, Bouner Denton M, Bates RB (1998) J Chromatogr A 827:45–55. doi:10.1016/S0021-9673(98)00755-9
Oka H, Ito Y, Matsumoto H (2000) J Chromatogr A 882:109–133. doi:10.1016/S0021-9673(99)01316-3
European Pharmacopoeia (2008) 6th edn
The United States Pharmacopoeia (USP26) (2003)
Hoogmartens J, Khan NH, Vanderhaeghe H, Van der Leeden AL, Oosterbaan M, Veld-Tulp GL et al (1989) J Pharm Biomed Anal 7:601–610. doi:10.1016/0731-7085(89)80226-2
Ding X, Mou S (2000) J Chromatogr A 897:205–214. doi:10.1016/S0021-9673(00)00779-2
Cherlet M, Schelkens M, Cronbles S, De Baker P (2003) Anal Chim Acta 492:199–213. doi:10.1016/S0003-2670(03)00341-6
Skùlason S, Ingòlfosson E, Kristmundsdòttir T (2003) J Pharm Biomed Anal 33:667–672. doi:10.1016/S0731-7085(03)00316-9
Oka H, Ikai Y, Ito Y, Hayakawa J, Harada K, Suzuki M et al (1997) J Chromatogr B Analyt Technol Biomed Life Sci 693:337–344. doi:10.1016/S0378-4347(97)00076-5
Yekkala R, Diana J, Adams E, Roets E, Hoogmartens J (2003) Chromatographia 58:313–316
Lykkeberg AK, Halling-Sorensen B, Cornett C, Tjornelund J, Hansen SH (2004) J Pharm Biomed Anal 34:325–332. doi:10.1016/S0731-7085(03)00500-4
Fiori J, Grassigli G, Filippi P, Gotti R, Cavrini V (2005) J Pharm Biomed Anal 37:979–985. doi:10.1016/j.jpba.2004.06.017
Monser L, Darghouth F (2000) J Pharm Biomed Anal 23:353–362. doi:10.1016/S0731-7085(00)00329-0
Nelis H, De Leenheer A (1980) J Chromatogr A 195:35–42. doi:10.1016/S0021-9673(00)81541-1
Böcker R (1980) J Chromatogr A 187:439–441. doi:10.1016/S0021-9673(00)80479-3
Hon J, Murray L (1982) J Liq Chromatogr 5:1973–1990. doi:10.1080/01483918208062867
European Pharmacopoeia Dissolution test for solid dosage forms, 01/2005:20903, V edn
European Pharmacopoeia Soya-bean oil, hydrogenated, 01/2005:1265 and Soya-bean oil refined, 01/2005:1473, V edn
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mancini, F., Cavallari, C., Filippi, P. et al. LC Analysis of Oxytetracycline and Chlortetracycline: Application for In Vitro Bio-Equivalence Study of Veterinary Medicines. Chroma 69, 215–220 (2009). https://doi.org/10.1365/s10337-008-0874-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0874-1