Journal of Pharmaceutics & Pharmacology
Download PDF
Research Article
Potential for the Use of Adamantanes for the Prevention and Treatment of the Neurological Complications of COVID-19
Butterworth Roger F*
University of Montreal, Canada
*Address for Correspondence: Butterworth Roger F, Professor of Medicine, University of Montreal, 45143 Cabot Trail, Englishtown, NS, B0C 1H0, Canada; E-mail: rb@enceph.com
Submission: 22 August 2020;
Accepted: 02 October 2020;
Published: 12 October 2020
Copyright: © 2020 Butterworth Roger F. This is an open access article
distributed under the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited.
Abstract
Widespread damage to the central and peripheral nervous systems
resulting from COVID-19 is becoming well established. Features
include impairments of the level [somnolence, stupor, coma] and
content [confusion, delirium] of consciousness, impaired senses of
taste, smell and vision as well as skeletal muscle manifestations. The
neuroinvasive nature of SARS-CoV-2 may contribute to the acute
respiratory failure of COVID-19. SARS-CoV-1 virus was detected in
the brain of infected patients along with neuronal necrosis and glial
hyperplasia. In SARS-CoV-2, modifications of crucial cellular pathways
[mitochondrial function, proteolysis, lipid metabolism] known to be
implicated in cellular aging and in neurodegenerative diseases occur.
Adamantanes, [amantadine and the structurally-related memantine]
are employed for the treatment of disorders of consciousness while
also manifesting effective antiviral properties. Clinical studies and Case
Reports at this early stage of COVID-19 reveal evidence of a protective
effect of amantadine in infected patients with benefit being ascribed to
amantadine’s effects on viral release into the host cell via mechanisms
involving the E channel of the virus or by the agent’s down-regulation of
the host protease Cathepsin L in addition to disruption of the lysosomal
pathway. Memantine has potent neuroprotective actions in both wellestablished neurodegenerative diseases as well as in viral disorders in
which it prevents neuronal cell loss and concomitantly reduces viral
replication in a dose-dependent manner. Controlled clinical trials for
the assessment of efficacy of these adamantanes for the prevention
and treatment of COVID-19 are now indicated.
Keywords
COVID-19; SARS-CoV-2; Adamantanes; Amantadine; Memantine; Disorders of consciousness; Neurodegeneration; Viral replication; Host cell proteases; Case reports; Clinical trials
Introduction
Reports of the involvement of the CNS in relation to COVID-19
continue to appear. In a review of 214 hospitalized patients from
Wuhan, China RT-PCR -confirmed diagnosis of COVID-19,
neurological symptoms occurred in 45.5% of those with severe
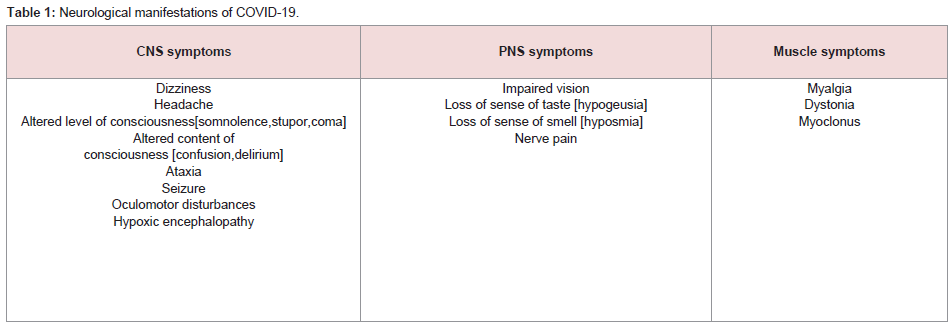
infection. Symptoms are generally classified into three categories
namely CNS manifestations [dizziness, headache, impaired
consciousness, acute cerebrovascular disease, ataxia, seizure], PNS
manifestations [impaired taste, smell or vision, nerve pain] and
skeletal muscle manifestations (Table 1). Impaired consciousness
consisted of two facets namely change of level of consciousness
[somnolence, stupor, coma] and content of consciousness [confusion,
delirium] (Table 1). Acute cerebrovascular included ischemic stroke
and cerebral haemorrhage diagnosed by clinical symptoms and CT
[1].
A subsequent retrospective study of 113 deceased patients
from Wuhan with COVID-19 described disorders of consciousness
ranging from somnolence to deep coma in one third of patients
and MRI evidence of a case of acute haemorrhagic necrotizing
encephalopathy was reported in an adult COVID-19 patient
consisting of haemorrhagic rim enhancing lesions in bilateral thalami,
medial temporal lobes and sub-insular regions [2,3], (Table 1).
A description of what is considered to be the first case of
meningitis/encephalitis in COVID-19 has appeared and it has been
suggested that the neuroinvasive potential of SARS-CoV-2 may
contribute to the pathogenesis of the respiratory failure characteristic
of COVID-19 [4,5].
Mechanisms involved in the pathogenesis of the CNS
manifestations of COVID-19 have not been definitively established
but the presence of pro-inflammatory biomarkers in these patients
suggests that SARS-CoV-2 related inflammatory mechanisms such as
a “cytokine storm” could be implicated [6].
Coronavirus-induced neurodegeneration:
Human coronaviruses [HCoVs] have well established
neuroinvasive and neurotropic properties. In an extensive search and
characterization of HCoV mRNA’s in human brain autopsy samples
from patients with a range of neurological diseases, a significantly
higher prevalence of the OC43 strain was noted in Multiple Sclerosis
[MS] patients. Three of four patients with Parkinson’s disease [PD]
showed increases of the 229E strain [7]. A previous study revealed
increases in Cerebrospinal Fluid [CSF] antibodies to coronaviruses
where responses to OC43 were greater than to 229E in PD patients
[8].Information relating to the cellular pathology and
pathophysiologic mechanisms implicated in the CNS consequences
of coronaviruses is largely derived from the results of studies in
experimental animal models. HCoV-OC43 can infect and may
persist in human neural cell lines with neuroinflammatory and
neurodegenerative consequences. The virus causes encephalitis in
susceptible mice and a single-point mutation in the viral spike protein
results in paralysis [9]. The neurotropic and neuroinvasive properties
of HCoC-OC43 were further characterized using an experimental animal model whereby virus inoculation of 21-day postnatal C57BL/6
and BALB/c mice manifested a generalized infection of the entire
CNS demonstrating neuroinvasiveness and neurovirulence targeting
neurons that showed vacuolation and degeneration. Damage was
judged to be the result of virus-mediated neuronal injury and it was
suggested that the prominent spongiform-like degeneration was
sufficient to trigger significant neuropathology in surviving animals
[9].
The SARS CoV-1 virus has been detected in the brains of patients
following the 2002-3 SARS epidemic accompanied by neuronal
necrosis, edema and glial hyperplasia. Infection of humans by
SARS-CoV-1 results in substantial morbidity and death primarily
from respiratory failure but the brain may also be affected resulting
in long-term neurological sequalae. The brain is also a major target
for infection in mice transgenic for human ACE-2, the receptor for
SARS-CoV-1 [10]. Infection of the brain is consistently observed
following intranasal inoculation in transgenic animals with brain
regions such as thalamus, cerebrum and brainstem being particularly
heavily infected. Death of infected animals appeared to be the result
of dysfunction or death of infected neurons especially those located in
cardio-respiratory centres in the medulla.
Given the rising body of evidence of destructive functional
and cellular CNS changes associated with the current COVID-19
pandemic, an important issue that has not been thoroughly addressed
relates to the long-term consequences to the health and quality of life
of survivors. To date, modifications of proteostasis, mitochondrial
function, lipid metabolism and stress responses have been identified
as crucial cellular pathways that are adversely affected by SARSCoV-2 and these pathways have been identified as those reported in
cellular aging and in neurodegenerative diseases such as PD [11].
Protective effects of adamantanes:
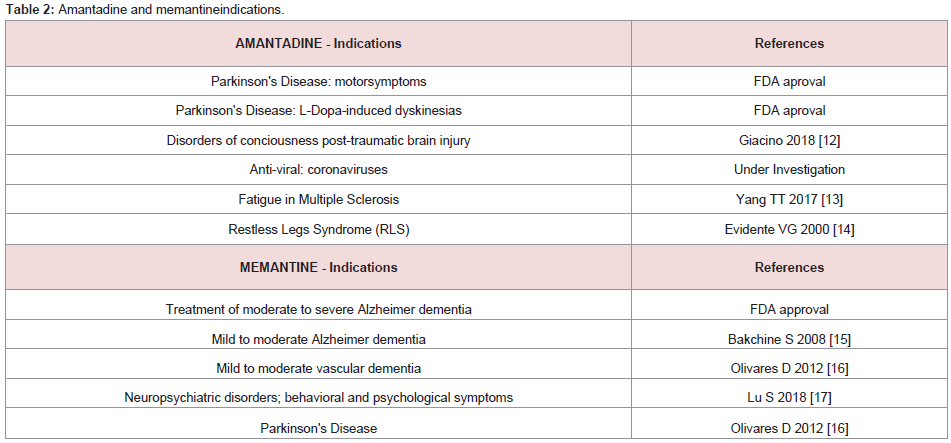
Amantadine and its structurally-related derivative memantine are
members of the adamantane family that are commonly-prescribed
for the treatment of CNS disorders (Table 2). Both have the ability
to cross the blood-brain barrier and both are potent non-competitive
antagonists of the NMDA receptor. Additionally, they each possess
a myriad of other properties and mechanisms of action related to coronaviruses and their effects on the [12-17], (Table 2).Amantadine:
Amantadine is an effective treatment for the motor disturbances
characteristic of PD and for the dyskinesias resulting from L-Dopa
in PD patients where its use results in a restoration of dopaminergic
transmission in basal ganglia by the re-establishment of the balance
between incoming nigrostriatal dopaminergic afferents with those of
striatal glutamatergic inputs from the cortico-spinal tract [18].Amantadine has also been found to be effective for the treatment
of disorders of cognition and of consciousness [DoC’s] resulting
from Traumatic Brain Injury [TBI] [19]. Again, the beneficial effects
of amantadine were judged to be the result of the stimulation of
the production of dopamine via dopa decarboxylase secondary to
NMDA receptor antagonism [20]. However alternative mechanisms
have been proposed. For example, there is evidence in support of the
notion that amantadine protects dopaminergic neurons by reducing
microglial activation while simultaneously stimulating the growth
factor GNDF in astroglia [21]. Either way, the evidence for the
efficacy of amantadine for the treatment of DoC’s has resulted in the
updating of American Academy of Neurology practice guidelines for
those disorders [12].
Of direct pertinence to the situation in COVID-19, DoC’s of
varying degrees of severity have consistently been reported during
both the Wuhan and European outbreaks of COVID-19 in severelyinfected patients [1,2,6,22]. Recommendations for the prevention
and treatment of DoC’s in COVID-19 have not yet been published
but, given the success of amantadine for treatment of DoC’s related
to TBI, perhaps amantadine could be considered. After all, given
the extent of neural damage attributed to SARS-CoV-2, perhaps
COVID-19 is, almost by definition, a traumatic brain injury.
Translational studies to the clinic related to the potential treatment
of COVID-19 have not yet appeared in the form of controlled
clinical trials. However, three case studies, although descriptive and
uncontrolled, provide evidence of beneficial effects of amantadine for
COVID-19. These cases consist of the following:
In a single case report, a 57-year-old man who tested positive for
SARS-CoV-2 by RT-PCR has been prescribed amantadine [100 mg
bid]. His asymptomatic wife [54 yrs] and daughter [33 yrs] who also
tested positive were prescribed amantadine [100 mg bid for 14 days]
as a preventive measure. The patient’s clinical status improved and
by day 6 he was able to breathe without oxygen supplementation and
was released on day 14. Neither of his family members developed
clinical symptoms of COVID-19 [23].
In a subsequent study, five PD patients, mean age 68+/- 15
yrs, tested positive for COVID-19 [by RT-PCR in upper and lower
respiratory specimens]. Infection was the result of person-to-person
contact with infected persons in all cases and all had received
amantadine [100 mg qid] for treatment of their PD for 3 months prior
to their exposure to the virus. This had been followed by a 2-week
quarantine. None of the 5 patients developed clinical manifestations
of COVID-19 and their PD symptoms remained unchanged [24].
The authors concluded that these observations may hold potential for amantadine to prevent COVID-19 in vulnerable patients.
Following up on the above report, a 75-year-old female patient
with PD of 16 years duration treated with medications for the
treatment of hyperthyroidism and stomach cancer in addition to
L-Dopa for her PD also received amantadine [100 mg/d]. Sometime
later, the patient’s husband showed classic symptoms of COVID-19
and he tested positive by RT-PCR. Bilateral pneumonia occurred
resulting in hospitalization and his death. 45 days later, the patient
had still not shown any signs of COVID-19 [25]. The author went
on to make a plea for further studies in PD patients on amantadine
therapy who become infected with SARS-CoV-2 in order to further
substantiate these findings.
Important advances have been made relating to the potential
mode of action of amantadine against SARS-CoV-2. One hypothesis
relates to the effect of amantadine which, upon entering the E channel
of the coronavirus, prevents release of the viral nucleus into the host
cell. Docking studies suggest an interaction of amantadine with the
amino acids ALA 22 and PHE 26 thus blocking the proton channel
[26]. An independent but contemporary investigation provides
evidence for a down-regulatory effect of amantadine on expression
of the host cell protease Cathepsin L in addition to disruption of the
lysosomal pathway resulting in interference with the capacity of the
SARS-CoV-2 virus to replicate [27].
Memantine:
It is generally accepted that glutamate [NMDA] receptormediated excitotoxicity is implicated in the pathogenesis of neuronal
cell death in a wide range of neurological and neurodegenerative
human conditions including Huntington’s disease, amyotropic lateral
sclerosis and multiple sclerosis. Comparable excitotoxic mechanisms
have also been proposed to explain the CNS consequences of viral
infections [28]. Primary neurons cultured in vitro and infected with
Rabies Virus [RABV] manifest severe neuronal damage that was
prevented by the addition of memantine [29]. In the same study,
memantine was found to extend the survival time of mice infected
with Japanese encephalitis virus [JEV] while decreasing the amount
of virus in the brain.In studies of neuroinvasive human respiratory coronaviruses,
a viral mutant of HCoV-OC43 with a single-point mutation in
the viral surface spike protein resulted in a paralytic disease that
implicated glutamate excitotoxicity [30]. Memantine treatment led to
improvements in motor performance, body weight loss and mortality
along with a reduction of viral replication in the CNS in a dosedependent manner. The authors suggested that memantine could be
useful as a prophylactic and therapeutic antiviral agent.
Case reports of clinical benefit of memantine for COVID-19
have started to appear in the literature. Seven patients with cognitive
impairment tested positive for SARS-CoV-2 by RT-PCR in upper
and lower respiratory specimens. Infection occurred following
person-to-person contact with infected individuals. All patients
had been receiving memantine [100 mg bid] for at least 3 months
prior to exposure to the virus and all had been quarantined for two
weeks since documented exposure. No patients went on to report any
clinical manifestations of COVID-19 and there were no significant changes in neurological status [24].
Conclusion
Significant damage to both central and peripheral nervous
systems resulting from COVID-19 is now widely established.
Consistent features include impairments of both the level and content
of consciousness, loss of sense of taste and smell, visionary loss, motor
incoordination and seizures. The neuroinvasive properties of SARSCoV-2 may contribute to the acute respiratory failure characteristic of
COVID-19 and modifications of mitochondrial function, proteolysis
and lipid metabolism characteristic of neurodegenerative diseases
have been shown to occur. Basic research in molecular virology has
identified mechanisms whereby members of the adamantine family of
agents such as amantadine and memantine have significant antiviral
properties with the capacity to impair replication of the virus. In
addition, their well-established neurobiological mechanisms such as
NMDA receptor antagonist actions are effective for the treatment of
motor dysfunction and disorders of consciousness associated with a
range of conditions including PD, traumatic brain injury as well as in
human coronaviral infections.
Clinical studies so far consist principally of Case Reports and
results are generally supportive of beneficial actions of adamantanes
for the prevention of COVID-19 in exposed individuals. In the
case of amantadine, benefit has been ascribed to its effects on
viral release into the host cell involving the E channel of the virus
or, alternatively/additionally to down-regulation and inhibition
of the host cell protease Cathepsin L and disruption of lysosomal
pathways. Memantine, on the other hand, has been shown to prevent
neuronal cell loss while concomitantly impairing viral replication in
a dose-dependent manner. Adequately-powered and appropriatelycontrolled clinical trials for the assessment of the efficacy and safety
of these and other adamantanes identified in the present document
for the prevention and treatment of COVID-19 are now indicated.
Acknowledgement
Research from the author’s Unit including costs of publication
of original articles and reviews was funded over the last two
decades by The Canadian Institutes of Health Research (CIHR)
and The Canadian Association for Study of The Liver (CASL).