H2O2/UV Photo-Oxidation of Gadung (Dioscorea Hispida Dennst.) Starch and its Product Physicochemical Characterization
Andri Cahyo Kumoro*, Diah Susetyo Retnowati, Ratnawati Ratnawati and Catarina Sri Budiyati
Department of Chemical Engineering, Faculty of Engineering Diponegoro University, SH Road, Tembalang-Semarang, INDONESIA 50275.
Corresponding Author E-mail: andrewkomoro@undip.ac.id
DOI : http://dx.doi.org/10.13005/ojc/320425
This work aimed to study the preparation and physicochemical properties characterization of oxidized gadung starch obtained from oxidation process implementing combined H2O2-UV irradiation. The photo-oxidation process was carried out in a well-mixed slurry reaction system at ambient temperature. The extent of oxidation was determined based on the carboxyl and the carbonyl contents of the oxidized gadung starch. The results show that oxidation process altered the carbonyl and carboxyl contents of the starch. Except the morphology of the starch, the swelling power, solubility and gelatinization temperature of the starch were severely affected by H2O2/UV oxidation process.
KEYWORDS:carbonyl; carboxyl; starch; oxidation; hydrogen peroxide; UV
Download this article as:| Copy the following to cite this article: Kumoro A. C, Retnowati D. S, Ratnawati R, Budiyati C. S. H2o2/Uv Photo-Oxidation of Gadung (Dioscorea Hispida Dennst.) Starch and its Product Physicochemical Characterization. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Kumoro A. C, Retnowati D. S, Ratnawati R, Budiyati C. S. H2o2/Uv Photo-Oxidation of Gadung (Dioscorea Hispida Dennst.) Starch and its Product Physicochemical Characterization. Orient J Chem 2016;32(4).Available from: http://www.orientjchem.org/?p=18848 |
Introduction
Low viscosity but high stability, clarity, film forming and binding properties has brought oxidized starch become one of important biomaterials in food and industrial applications1. The food products where oxidized starch is used are neutral tasting and low viscosity such as a lemon curd, salad cream and mayonnaise2. Oxidized starch can be used as a coating and sealing agent in confectionary, as an emulsifier3, as a dough conditioner for bread4, as a gum Arabic replacer5 and as a binding agent in batter applications6. The permitted carboxyl content of oxidized food starches may range up to 1.1%, which corresponds to the level found in the most highly oxidized type having roughly a 90 fluidity7.
Several methods have been used to prepare oxidized starch, which include the use of oxidizing agent such as hydrogen peroxide, air oxygen, ozone, bromine, chromic acid, permanganate, nitrogen dioxide and hypochlorite1. However, hypochlorite oxidation is the oldest and most popular method for commercial scale production of oxidized starches for food applications8. The extent of oxidation of starch is affected by many factors, such as temperature and pH9, type and amount of catalyst10, oxidant dosage, starch: liquor ratio, time11 and starch source and molecular structure12.
From environmental point of view, hydrogen peroxide is the most favorable oxidizing agent. The mechanisms of reaction between hydrogen peroxide with starch are very complex and have been reported to proceed via a radical chain reaction. In addition to carbonyl as a primary functional group produced in the peroxide-oxidized starches, a minor amount of carboxyl group was also formed. However, hydrogen peroxide oxidation of starch requires long reaction time, high temperature and/or high pH13. Hydrogen peroxide does not oxidize starch at ambient temperatures but in boiling solutions to produce dextrin and glucose14. To overcome this shortcoming, an attempt on oxidation of starch using H2O2 in the presence of UV had been carried out and successfully produced oxidized starch with significant carboxyl and carbonyl contents15. The use of UV light as reaction promoter is a better alternative to heavy metal with regard to considerable heavy metals residue in the oxidized starch16. The combined UV/H2O2 process can be carried out under ambient conditions17. In this process, the photolysis of H2O2 by UV light effectively generates hydroxyl radicals18. Although a very powerful oxidizer, this radical is not selective19. This highly reactive radical readily reacts with carbohydrate by abstracting hydrogen from a C–H group on the sugar ring, forming a radical (R•CHOH) which further undergoes acid or base catalyzed rearrangement resulting in cleavage of glycosidic bond and a carbonyl group20. Higher carboxyl and carbonyl contents can be obtained under acidic rather than alkaline conditions, increasing concentrations of H2O2 and absence of oxygen15. Under alkaline conditions, carbohydrates having a free or potentially free carbonyl group may undergo further reactions via various pathways; some of which afford a carboxyl group21.
As far as literatures survey being conducted, no work has been reported in the oxidation of gadung (Dioscorea hispida Dennst) starch using H2O2/UV irradiation. This work aimed to study the preparation and physicochemical properties characterization of oxidized gadung starch obtained from oxidation process involving H2O2/UV irradiation. Factors affecting the photo-oxidation process, such as gadung starch slurry consistency (GSC) and hydrogen peroxide concentration were investigated in a well-mixed slurry reaction system.
Materials and Methods
Materials
Matured gadung tubers were harvested from Klaten District, Central Java Province, Indonesia. They were washed with flowing water to remove dirt, peeled, sliced into chips and detoxified using the method suggested by Kumoro et al. with slight modification22, 23. The reagent-grade 35 % w/w hydrogen peroxide stock solution and other chemicals of analytical grade (purity ³ 98% w/w) used in this work were purchased from Sigma-Aldrich Pte. Ltd, Singapore. All of the chemicals were used as received without pretreatment.
Starch extraction
The detoxified gadung chips were pulverized using a kitchen blender (Panasonic MX-900M) for 3 min. An adequate amount of distilled water was added to the pulp and the slurry was stirred for 30 minutes before being filtered using cheesecloth to separate the coarse fiber. The suspension was centrifuged at 5000×g for 10 min, and followed by decantation of the supernatant. The sediment was re-suspended in distilled water, after which being centrifuged as previously described. Finally, the sediment was oven dried at 50oC for 12 h, ground, and sieved through a 300 mesh sifter. The starch was stored in a sealed plastic bag and kept in a desiccator for further use.
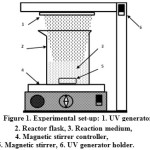 |
Figure 1: Experimental set-up: 1. UV generator 2. Reactor flask, 3. Reaction medium, 4. Magnetic stirrer controller, 5. Magnetic stirrer, 6. UV generator holder. |
Photo-oxidation of gadung starch
The photo-oxidation reactor used in this work was a 500 mL beaker glass equipped with a quartz immersion well containing a 450 watt Heraus monochromatic UV lamp, which emits at a wavelength of 254 nm (Fig. 1). To prevent any leakages of UV light, the whole system was covered with aluminum foil. Prior to photo-oxidation, a predetermined mass of native gadung starch (NGS) was dispersed in 250 mL of distilled water by vigorous stirring in the reactor using a magnetic stirrer to obtain starch-water slurry with certain consistency. Then, a predetermined amount of H2O2 was introduced drop wise to the slurry under continuous stirring until complete mixing of the whole contents. The reaction mixture was photolyzed by turning on the UV lamp and the whole contents were kept at ambient temperature for 2 hours under continuous stirring at pH 5. After reaction completed, the slurry was withdrawn from the reaction system and filtered in vacuo by which a creamy solid cake was obtained. After washing with water and followed by appropriate drying, the oxidized starch was obtained as a white solid. The oxidized starch was then subjected to carbonyl and carboxyl content determinations. In addition, the swelling power, solubility, gelatinization temperature, and structure of the oxidized starch obtained were also determined.
Physicochemical properties analysis
The carboxyl contents of the oxidized flour were determined by paste titration method5. The hydroxylamine hydrochloride method was used to estimate the total carbonyl contents24, while the amylose content was analyzed by method developed by Juliano25. The analyses were conducted in triplicates. Swelling power and solubility of the starch were determined according to the methods of Tattiyakul et al.26. Thermal characteristics of native and oxidized gadung starches were studied by using a differential scanning calorimeter (DSC; Mettler Toledo, Switzerland) as previously used by Sandhu and Singh27. Granules morphology of the starch samples was studied with a scanning electron microscope (SEM) (JEOL JSM-5310, England) according to Sangseethong et al.28.
Results and Discussion
Effect of H2O2 concentration
In this work, the extent of oxidation is determined based on the carboxyl and carbonyl group increase resulted from dual actions of hydrogen peroxide and UV light. Hydrogen peroxide concentration is an important parameter for the oxidation of organic compounds in the UV/H2O2 photoreactor. Indeed, the hydroxyl free radicals produced upon photolysis of H2O2 can react with anhydroglucose of starch molecules. The changes of amylose, carbonyl and carboxyl contents of gadung starches before and after oxidation process are presented in Table 1.
As shown in Table 1, hydrogen peroxide oxidation of gadung starch without UV irradiation almost did not change carbonyl, carboxyl and amylose contents. Previous researchers reported that direct oxidation of organic compounds by atmospheric oxygen from decomposition of hydrogen peroxide is very slow29. Similarly, oxidation of gadung starch by UV irradiation alone (0 % H2O2) was considered negligible due to low quantum yield of the starch particles to hydrogen peroxide30.
Table 1: Amylose, carbonyl and carboxyl contents of native and oxidized gadung starches obtained from various reaction conditions
|
Material/ Reaction Condition (GSC; H2O2 Conc.) |
Amylose (%) |
Carbonyl (%) |
Carboxyl (%) |
|
NGS |
34.72±0.50 |
0.43±0.20 |
0 |
|
GS H2O2, no UV |
34.69±0.10 |
0.45±0.10 |
0.09±0.05 |
|
GS H2O2 + UV |
|||
|
15 % w/v; 0 % H2O2 |
34.70±0.3 |
0.43±0.03 |
0.03±0.02 |
|
15 % w/v; 1 % H2O2 |
34.26±0.1 |
1.34±0.05 |
0.85±0.20 |
|
15 % w/v; 2 % H2O2 |
32.73±0.1 |
1.55±0.10 |
1.09±0.05 |
|
15 % w/v; 3 % H2O2 |
32.29±0.5 |
1.82±0.05 |
1.38±0.20 |
|
15 % w/v; 4 % H2O2 |
32.46±0.2 |
1.43±0.10 |
1.75±0.10 |
|
10 % w/v; 2 % H2O2 |
32.65±0.5 |
1.53±0.20 |
1.16±0.05 |
|
20 % w/v; 2 % H2O2 |
32.93±0.3 |
1.49±0.05 |
1.05±0.10 |
|
25 % w/v; 2 % H2O2 |
34.07±0.5 |
1.35±0.15 |
0.93±0.10 |
|
30 % w/v; 2 % H2O2 |
34.19±0.1 |
1.16±0.15 |
0.88±0.20 |
Under UV irradiation, the carbonyl content increases significantly by increasing H2O2 concentration up to 3% w/v, but then decreases. In contrast, the carboxyl content increases steadily by increasing H2O2 concentration. The increase in the extent of oxidation of starch with increasing H2O2 concentration, as shown by the carboxyl and carbonyl contents, indicates a progressive increase in the creation of oxidizing species (hydroxyl radicals). At low H2O2 concentration, H2O2 cannot generate enough hydroxyl radicals and the oxidation rate is consequently slow. Therefore, most of the free hydroxyl radicals are directly consumed by anhydroglucose of gadung starch molecules.
In the presence of high concentration of H2O2, the free hydroxyl radicals preferentially react with the excess of H2O2 and thus competing with gadung starch. This explains why excessive H2O2 concentration may inhibit the degradation of gadung starch19. The decrease in the carbonyl content when oxidation was carried out at H2O2 concentration higher than 3% w/v indicates that, (a) the oxidizing species (free radicals and nascent oxygen) are so abundant that they mutually destroy themselves by coupling19, (b) carbonyl groups formed at higher H2O2 concentration are converted to carboxyl groups12, which finally, are lost via decarboxylation, and/or (c) water soluble highly oxidized starches with very short chains are formed and being dissolved in the reaction medium11.
Oxidation causes a significant reduction in amylose content of oxidized gadung starch. Similar observation was reported for corn starches6. The decreasing amylose content might be due to depolymerization of starch molecules into the polymer chain with chain lengths shorter in greater numbers12.
Effect of gadung starch consistency (GSC)
It is clear in Table 1 that the carboxyl content decreases as the GSC value increases within the range studied. On the other hand, the carbonyl content increases on increasing GSC value from up to 15 %. Under this condition, the starch particles move easily in the slurry and the creation of hydroxyl radical via H2O2 photolysis is still high18.
Further increase in GSC value is accompanied by a substantial decrease in carbonyl content, most probably as a result of lower availability of hydroxyl radical in the reaction medium and the increase of viscosity of the reaction slurry. At higher GSC, more starch particles present in the reaction medium, which hinder the absorption of UV light by H2O2 solution, and in turn reduce the formation of hydroxyl radicals. Concerning the safety regulation by EU scientific committee for food and technical point of views, the best reaction condition is photo-oxidation using GSC of 15 % w/v and 2 % H2O2 concentration by which 1.09 % carboxyl content in oxidized starch is achieved.
Swelling power and solubility
As seen in Table 2, the swelling power of gadung starch decreased after being oxidized using dual action of H2O2/UV, whereas the solubility increased. The reduction in swelling power and increase in solubility of the starch after oxidation may come as a result of depolimerization and structural disintegration within the granules of the starch during the process of modification2. These phenomena can also be correlated with the changes in amylose to amylopectin ratio of the starch31.
Temperature and enthalpy of gelatinization
Table 2 shows that gelatinization transition temperatures (To, Tp and Tc) of oxidized starches were higher than that of native starch. Similar results were reported previously for cassava starch28. The increased in the To, Tp and Tc of starches upon oxidation might also be due to the depolymerization in the amorphous region which functions to destabilize the crystalline lamella. Once the amorphous regions were degraded, their destabilizing effect on the crystalline domains was destroyed. Therefore, the gelatinization of the resulting starch occurred at a higher temperature28.
Table 2: Some physical properties of native and selected oxidized gadung starches
|
Material |
Swelling power (g/g) |
Solubility (g/100g) |
Gelatinization Temperature |
DHGel |
|||
|
To (oC) |
Tp (oC) |
Tc (oC) |
(J/g) |
||||
|
NGS |
4.67±0.2 |
6.53±0.1 |
71.54±1.2 |
76.35±1.5 |
85.03±0.6 |
16.12±1.9 |
|
|
GS H2O2, no UV |
4.59±0.0 |
6.64±0.5 |
74.13±1.0 |
79.45±1.3 |
94.29±0.9 |
14.41±1.5 |
|
|
GS H2O2 + UV |
3.81±0.1 |
9.77±0.4 |
76.94±1.5 |
82.47±1.1 |
96.51±2.0 |
13.85±2.1 |
|
In contrast to transition temperatures, coupled UV/H2O2 oxidation reduced the gelatinization enthalpies (DHGel) of gadung starch. The decrease of DHGel value indicated that oxidation caused a weakening of the starch granules, probably from the partial degradation of starch molecules in the crystalline lamellae. Consequently, less energy was needed to gelatinize starch6.
Scanning electron microscopy (SEM)
In this study, no noticeable differences were observed between the appearances of the native and the oxidized gadung starch granules. The scanning electron micrographs show that native gadung starch granules are polyhedral in shape with surface-average diameter of about 4.0 mm. The starch granules are smooth-surfaced with no evidence of any crevices (Fig. 2a). Similar observation was also reported by Tattiyakul et al. 26, 32.
 |
Figure 2: Micrographs of gadung starch particles(a) native, (b) oxidized by H2O2 without irradiation and(c) oxidized by H2O2 + UV irradiation |
In general, no obvious changes or signs of damages on the granule surface of oxidized gadung starch obtained from H2O2 oxidation without UV irradiation (Fig. 2b). Similar results regarding changes in granule morphology has been reported previously2, where potato, corn and rice starches granules remained intact after the modification with hypochlorite at the levels of 0.8 and 2% active chlorine. Although the oxidized starch in this work retains its polyhedral shape, a slightly roughened surface was observed for the starch granules oxidized with H2O2 irradiated by UV light (Fig. 2c), presumably due to a localized extensive oxidation. This is an indication that the oxidation took place from the surface of the granules first and later in the interior of the granule33.
Conclusions
The oxidation of gadung tuber starch using H2O2/UV oxidation process altered its carbonyl, carboxyl and amylose contents. In addition, the swelling power, solubility and gelatinization temperature of the starch were also affected by H2O2/UV oxidation process. However, the structure of gadung starch granules was not significantly altered by the H2O2/UV oxidation process.
Acknowledgements
The authors greatly acknowledge the Ministry of Research, Technology and Higher Education of the Republic of Indonesia for its financial support through National Strategic Research Grant under contract No. 148-03/UN7.5.1/PG/2015.
References
- Lawal, O. S.; Adebowale, K. O.; Ogunsanwo, B. M.; Barba, L. L.; Ilo, N. S. (2005). Int. J. Biol. Macromol. 2005, 35, 71–79
CrossRef - Lawal, O. S. Food Chem. 2004, 87, 205–218.
CrossRef - Konoo, S.; Ogawa, H.; Mizuno, H.; Iso, N. J. Jpn. Soc. Food Sci. Technol. 1996, 43, 880–886
CrossRef - Mazur, P. Y.; Stolyarova, L. I.; Muraschkina, L. V.; Dyatlov, V. A. Emaehrung 1989, 13, 155–156
- Chattopadhyay, S.; Singhal, R. S.; Kulkarni, P. R. Carbohydr. Polym. 1997, 34, 203–212
CrossRef - Sandhu, K. S.; Kaur, M.; Singh, N.; Lim, S. T. LWT – Food Sci. Technol. 2008, 41, 1000–1010
CrossRef - Wurzburg, O. B., Modified Starches, CRC Press. Boca Raton, (2006)
- Wurzburg, O. B., Converted Starches, CRC Press. Boca Raton, (1986)
- Wang, Y. J.; Wang, L. Carbohydr. Polym. 2003, 52, 207–217
CrossRef - Tolvanen, P.; Sorokin, A.; Ma¨ki-Arvela, P.; Leveneur, A.; Murzin, D. Y.; Salmi, T. Ind. Eng. Chem. Res. 2011, 50, 749–757
CrossRef - El-Sheikh, M. A.; Ramadan, M. A.; El-Shafie, A. Carbohydr. Polym. 2010, 80, 266–269
CrossRef - Kuakpetoon, D.; Wang, Y. J. Carbohydr. Res. 2006, 341, 1896–1915
CrossRef - Hage, R.; Lienke, A. J. Mol. Catal. A: Chemical 2006, 251, 150–158
CrossRef - Harmon, R. E.; Gupta, S. K.; Johnson, J. Starch 1971, 23, 347–349
CrossRef - Harmon, R. E.; Gupta, S. K.; Johnson, J. Starch 1972, 24, 8–11
CrossRef - Tolvanen, P.; Sorokin, A.; Ma¨ki-Arvela, P.; Murzin, D. Y.; Salmi, T. Ind. Eng. Chem. Res. 2013, 52, 9351−9358
CrossRef - Liu, P.; Li, C.; Liang, X.; Xu, J.; Lu, G.; Ji, F. Environ. Technol. 2013, 34(15), 2231-2239
CrossRef - Chelme-Ayala, P.; El-Din, M. G.; Smith, D. W. Water Res. 2010, 44, 2221–2228
CrossRef - Aleboyeh, A.; Moussa, Y.; Aleboyeh, H. Dyes Pigm. 2005, 66,129-134
CrossRef - Arts, S. J. H. F.; Mombarg, E. J. M.; van Bekkum, H.; Sheldon, R. A. Synthesis 1997, 6, 597-613
CrossRef - Isbell, H. S.; Frush, H. L. Carbohydr. Res. 1987, 161, 181–193
CrossRef - Kumoro, A. C.; Retnowati, D. S.; Budiyati, C. S. J. Appl. Sci. Res. 2011, 7(12), 2140–2146
- Kumoro, A. C.; Amalia, R.; Budiyati, C. S.; Retnowati, D. S.; Ratnawati, R . J. Food Sci. Technol. 2015, 52(10), 6615-6622
CrossRef - Juliano, B. O. Cereal Sci. Today 1971, 16, 334–336
- Smith, R. J., Characterization and analysis of starches, Vol. II.; Academic Press. New York, (1967)
- Tattiyakul, J.; Naksriarporn, T.; Pradipasena, P. Food Bioprocess Tech. 2012, 5 (3), 964–971
CrossRef - Sandhu, K. S.; Singh, N. Food Chem. 2007, 101, 1516–1524
CrossRef - Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Carbohydr. Polym. 2010, 82, 446–453
CrossRef - Legrini, O.; Oliveros, E.; Braun, M. Chem. Rev. 1993, 93, 671-689
CrossRef - Alnaizy, A; Akgerman, A. Adv. Environ. Res. 2000, 4, 233-244
CrossRef - Udachan, S.; Iranna, A. K.; Hend, S. G. M. Int. Food Res. J. 2012, 19, 315-319
- Tattiyakul, J.; Naksriarporn, T.; Pradipasena, P.; Miyawaki, O. Stärke 2006, 58, 170–176
CrossRef - Kachkarova-Sorokina, S. L.; Gallezot, P.; Sorokin, A. B. Chem. Commun. 2004, 24, 2844-2845.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









