Manuscript accepted on : 17-03-2020
Published online on: 25-03-2020
Plagiarism Check: Yes
Reviewed by: Biswaranjan Paital ![]()
![]()
Second Review by: Prithi Shanthi
Final Approval by: Dr. Msafiri Yusuph Mkonda ![]()
![]()
Protein Antioxidant Capacity from Moringa Oleifera Fresh and Commercialised Leaf
Zulkifli Z. A1 and Rahmat Z.1,2*
1Department of Biosciences, Faculty of Science, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia
2Institute of Bioproduct Development, Universiti Teknologi Malaysia, 81310 Johor-Bahru, Johor, Malaysia
Corresponding Author E-mail: zaidahrahmat@utm.my
DOI : http://dx.doi.org/10.13005/bbra/2820
ABSTRACT: Moringa oleifera is one of the most reported medicinal plants with various health benefits while its commercialised leaf in dried and powdered form is currently a blooming herbal product in the market. Apart from some profiling work, the protein from M. oleifera that tops other plants was never focused. Since protein is an essential nutrient and could interact with a substrate or another protein, its role in the pharmacological activity is highly anticipated. Hence, this study was done to highlight on the antioxidant ability of protein and comparing it with crude extract from fresh and commercialised Moringa’s leaf via Ferric Reducing Antioxidant Power (FRAP) and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical assay. Interestingly, the protein extract from commercialised leaf of M. oleifera elucidated significantly higher antioxidant activity compared to other samples. It had the highest reducing power (±SEM) of 2381.88±25.16 (mM Fe2+/g) and even highest radical scavenging activity of 46.26±0.2 (%RSA). Thus, the protein contained in this blooming product would benefit its consumers. Plus, instead of relying solely on the crude extract, detailed study on the mechanism of the protein extracts on its therapeutic properties is highly anticipated.
KEYWORDS: Antioxidant; Moringa Oleifera Leaf; Protein
Download this article as:| Copy the following to cite this article: Zulkifli Z. A, Rahmat Z. Protein Antioxidant Capacity from Moringa Oleifera Fresh and Commercialised Leaf. Biosci Biotech Res Asia 2020;17(1). |
| Copy the following to cite this URL: Zulkifli Z. A, Rahmat Z. Protein Antioxidant Capacity from Moringa Oleifera Fresh and Commercialised Leaf.Biosci Biotech Res Asia 2020;17(1). Available from: https://bit.ly/2JcQ5Ev |
Introduction
Nowadays, our modern society favour the traditional alternative medicine which practically involve herbal-based product. This natural option had won consumers heart over synthetic chemicals that require sophisticated skills and technologies. Proven medicinal traits brought by medicinal plants was the major reason for the trend. Moringa oleifera or drumstick tree is one of them. It is a native medicinal plant of Southeast Asia which had conquered the ancient medicine world since our ancestor’s time1. Since M. oleifera, is considered as nature’s gold, scientists were really keen to explore this plant2. Countless study on their pharmacological properties had been reported which includes the anticancer, antidiabetic, antibacterial, and antioxidant activity and the list goes on and on3-7. Prominently, these scientific proofs led to the blooming of Moringa-based product in the market especially its highly demanded leaf. For the sake of the consumers, side by side comparison of fresh and commercialised leaf had to be revealed.
On top of that, health conscious society we are currently living in are highly aware of the importance and benefits of antioxidants. These antioxidants are highly responsible in reducing and scavenging free radicals that could harm our precious body8. Besides, antioxidant has the ability to prevent oxidation and damages caused by the oxidation process itself9. The main reason of the trending study on the application of medicinal plant including M. oleifera around the world is the antioxidants activity of phytochemical compounds present in the plant10. Therefore, scientist had been extremely busy reporting on the in vivo and in vitro antioxidant activity of M. oleifera11. Their studies had covered various extract from the plant including water and crude extract of the plant leaf and extract from different fraction and maturity stage of the plant leaf 12-14. However, no one had ever tested on the antioxidant activity of the protein extracted from the plant. Even though protein is claimed to be higher in this plant compared to even egg and yogurt 15, the protein’s bioactivity such as antioxidant activity is yet to be revealed. In fact, since protein is an essential nutrient needed by every living organism including human, its potential role in pharmacological traits including antioxidant activity is highly anticipated. Prominently, antioxidant agent from Moringa plant is free from animal’s cholesterol hence reducing risk of rejection and suits the vegetarian. This plant is also highly abundance in nature and can be widely cultivated to support mass production for market supplies.
Materials and Methods
Samples Preparation
The fresh leaf of M. oleifera was collected in Johor Bahru, Malaysia and the commercialised leaf bought from a company in Sarawak, Malaysia. Fresh leaf from the first five branches was removed manually, weighed at 500mg before it was ground with liquid nitrogen. Meanwhile, grinding was not applied to the commercialised leaf since it is already sun dried and in powdered form. It was weighed at 100mg as recommended by the manufacturer, where 100mg is equivalent to 500mg fresh leaf.
Protein Extraction
1mL extraction buffer consisting of 100mM Tris HCL pH 7.5, DTT and PMSF was added. The mixture was then vortexed and centrifuged at 16000g in 4oC for 15 minutes. Next, the supernatant was transferred to new tube before it was centrifuged again. The process was repeated until the supernatant was free of debris. The protein quantity and quality were then checked via Bradford assay and 1D SDS-PAGE respectively. The Bradford assay was chosen since it offers simple and sensitive technique with fewer interfering from other substances such as salts, solvents, and buffers 16.
Methanolic Extraction
Triplicate of 1g ground fresh and commercialised leaf of M. oleifera was added with 30mL 80% (v/v) methanol. The mixtures were then left for overnight agitation at 100-120 rpm at room temperature. The next day, the mixtures was filtered before another 20mL 80% (v/v) methanol was added. The new mixture was then left for overnight agitation at 100-120 rpm at room temperature. The mixture was filtered the next day and the filtrate was pooled with the one from the previous day. The filtrate then undergo rotary evaporation until it was fully dried. The dried mixture was then dissolved with pure DMSO before it was used for the antioxidant assay.
Antioxidant Assay
FRAP Assay
The FRAP reagent was prepared freshly at the time of use prior to addition of 90μL ultrapure water and 30μL of the crude extract and control or blank. However only 0.75μL of the protein extract were used which is 40 times diluted from the crude extract since the protein product is pure compared to the crude extract. Next, the mixture was incubated at 37oC for 30 minutes in the incubator before its absorbance was read at 593nm against the blank. The calibration curve, comprising of methanolic solution of known Fe (II) concentration ranging from 100 to 2000 μmol/L was prepared. The regression equation from the generated calibration curve was used to calculate the FRAP values (Fe 2+/g) for each sample triplicate.
DPPH Assay
The assay used 20μL of protein, crude extract sample and control before addition of 1.48μL of freshly prepared 0.1mM DPPH solution in methanol. The reaction was then allowed to stand at 37oC for about 20 minutes. After that, the absorbance reading was taken at 517nm before the percentage of the sample’s radical scavenging activity (RSA) was calculated by using this formula of %RSA= ([Abs control-Abs sample]/Abs control) x100.
Statistical Analysis
The results were analysed by one-way analysis of variance (ANOVA) by SPSS Statistics software to compare the significant differences of the extracts. The data were expressed as mean ± SEM where the difference was considered significant when the P value is less than 0.05.
Results and Discussion
Protein Analysis
The protein quantity of M. oleifera leaf is presented as in Table 1 and the quality as in Figure 1. Protein analysis of the fresh and commercialised leaf executed in this study showed that fresh leaf confers better protein quality and quantity. According to Table 1, the fresh leaf has better protein concentration, total protein amount, and even protein yield at 1448.95±4.11 (µg/mL) ± SEM, 72.45±0.21 (µg) ± SEM, and 0.14±0.00 (µg/mg) ± SEM respectively. This is in comparison with the commercialised leaf that exhibit protein concentration, total protein amount, and protein yield of 644.35±17.54, 32.22±0.88, and 0.06±0.00 respectively. Additionally, according to Figure 1, the protein electrophoretic pattern of the fresh leaf was better with more visible bands at lower molecular weight and higher band intensity.
Table 1: Protein quantification of the fresh and commercialised leaf via Bradford assay.
| Samples | Final Concentration (µg/mL) ± SEM | Total Protein Amount
(µg) ± SEM |
Protein Yield (µg/mg) ± SEM |
| Fresh leaf | 1448.95±4.11 | 72.45±0.21 | 0.14±0.00 |
| Commercialised leaf | 644.35±17.54 | 32.22±0.88 | 0.06±0.00 |
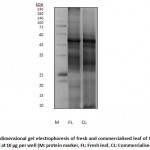 |
Figure 1: One dimensional gel electrophoresis of fresh and commercialised leaf |
More intense protein bands of both low and high molecular weight were observed in the fresh leaf compared to the commercialised leaf. Still, both fresh and commercialised leaf share certain similarities of distinct band formation especially at higher molecular weight for an instance at 40-50 kDa size. The intense band formation at 50kDa highly reflects the abundance of the 50kDa protein in this plant’s leaf. In fact, that individual band is expected to be the large subunit of Ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCo). It is the most abundant protein on earth and plays crucial role as the central enzyme in photosynthesis and carbon fixation 17. Furthermore, more intense protein bands of both low and high molecular weight were observed in the fresh leaf compared to the commercialised leaf.
Total Antioxidant Activity
In this study, antioxidant activity of protein and crude extract from both fresh and commercialised leaf of this medicinal plant was analysed via FRAP and DPPH assays as shown in Table 2. Different weight of the starting material was used to obtain the two types of extract involved in the antioxidant assays where 0.5g and 1g leaf were utilised to prepare the protein and methanolic extract respectively. This is due to the optimisation of the starting material (protein weight) according to the quality of the protein.
Table 2: Total antioxidant activity of protein and crude extract from fresh and commercialised leaf of M. oleifera
| Samples | FRAP(mM Fe2+/g) | DPPH (%RSA) |
| PE-FL1 | 2381.88±25.16a | 46.26±0.25a |
| PE-CL2 | 3044.51±11.51b | 88.32±0.03b |
| ME-FL3 | 920.01±31.88c | 84.05±0.7c |
| ME-CL4 | 1752.38±19.47d | 85.73±0.21d |
1Protein Extract-Fresh Leaf, 2 Protein Extract-Commercialised Leaf, 3Methanolic Extract-Fresh Leaf, 4 Methanolic Extract-Commercialised Leaf. The values are means (n=3) ± SD where values with different superscript are significantly different at p<0.05.
According to the FRAP assay findings, there is a significant difference (P<0.05) between the protein and crude extract from both fresh and commercialised leaf. Protein extracted from both leaf type exhibited higher FRAP values of 2381.88±25.16 and 3044.51±11.51 (mM Fe2+/g) respectively in comparison with its methanolic extract with only 920.01±31.88 and 1752.38±19.47 mM/g of ferrous ion detected in the sample.
Higher FRAP values conferred by the protein extract implies better reducing power compared to the crude extract even though lower starting material (weight) was used. In fact, high reducing power of the protein extract was observed in both leaf types. Interestingly, the highest ferrous ion concentration was found in the protein extract of the commercialised leaf.
On the other hand, the DPPH assay tested on these four samples had produced different pattern compared to the FRAP assay. This assay was executed in order to determine the scavenging ability of the samples while FRAP assay was able to discover their reducing power, hence the dissimilarity of the findings are expected. According to the finding via DPPH assay, there is a significant difference (P<0.05) between the protein and crude extract of the fresh and commercialised leaf. The lowest scavenging ability was conferred by protein extracted from the fresh leaf with only 46.26±0.25 percent of radical scavenging activity. Meanwhile, the best radical scavenging activity of 88.32±0.03 percent was exerted by protein extract of commercialised leaf followed by methanolic extract of commercialised leaf and fresh leaf at 85.73±0.21 and 84.05±0.7 percent respectively. However, bear in mind that the protein extract used lower starting material weight of the methanolic extract. Hence, theoretically the protein extract still had the best radical scavenging activity compared to the methanolic extract.
Prominently, according to both antioxidant assays tested on the samples, the protein extracted from the leaf of Moringa manifests promising values especially in their reducing power. Although the protein quality of the fresh leaf was better than the commercialised leaf, the antioxidant assay performed on these two leaf types reveal that the protein of the commercialised leaf confers not only better but the best reducing and scavenging abilities.
Interestingly, the processing procedure applied to the commercialised leaf had possibly enhanced its antioxidant properties. According to Dipika and Krishna, (2010) 18 variation in the drying method and temperature applied to the plant part will affect its content differently. In fact, nutrient content including protein from different plant parts react and respond differently when processing is applied to them since they show variation of physical and chemical properties.
Nowadays, processing involving boiling, drying, and roasting were mostly compulsory in producing food products for consumers. These processing applied to the food component were intended to remove the antinutrients present such as phytate, tannins, and lectins in plants seed, root and even leaf. However, the processing applied might jeopardize the nutritional content, especially availability of the protein in the food itself. This is proven by the low value of the protein digestibility, fractions and extractability after severe heat treatment in the seed of African breadfruit 19.
Furthermore, most of the studies involving the effect of drying or processing to the plant prove that the processing applied did reduce and denature the plant’s nutrient and content20. However, not only the processing applied to different sample affect them differently18. A study done by Ayegba et al., (2017) reported that M. oleifera leaf is best dried at a temperature of 60ºC with the least loss of vitamins and protein content in comparison to other drying methods and temperature21. Interestingly, 60 oC is more than the melting point of most protein. The ability of the commercialised leaf to confer higher antioxidant activity compared to the fresh type is most likely due to the processing applied to the leaf by the manufacturer where the plant’s content and nutrients were still maintained. Hence, the most demanded Moringa’s leaf in the market such as the dried and powder types are actually benefiting its consumers not only in providing easier handling and storage but also better antioxidant power compared to the fresh type.
Antioxidant Analysis of Protein
Even though no study on pharmacological activity such as antioxidant of the protein extracted from M. oleifera had been reported, multiple studies on the antioxidant activity of the protein extracted from other plant and food had been published. In comparison with other study, the antioxidant activity analysis on the protein of several processed food such as chickpeas and Petrovac sausage had also led to promising potential of the protein22, 23. In other words, our study suggests that processed food such as leaf of Moringa conferred better antioxidant activity than the fresh. In fact, processed food is also suggested as natural thermostable antioxidant food with enhanced antioxidant activity after the processing applied to them.
Furthermore, hydrolysed protein with higher number of the smaller peptide formation compared to the total protein was claimed to have better bioavailability. According to Agyei et al. (2018), peptide offer high specificity, strong binding affinity and even high stability24. This is probably due its low molecular weight which enables it to reach target easily. Interestingly, most of the reported bioactive peptides including the antioxidant peptides were small peptides with low molecular weight25, 26. Therefore, several studies on the antioxidant activity of the hydrolysed protein had been reported. Protein hydrolysate of Ficus deltoidea, alfalfa leaf, and canola were proven to exhibit high reducing power and radical scavenging activity27-29. Thus, the antioxidant activity of protein have better advantage to serve the mankind.
Currently, there are more than 700 antioxidant proteins in the antioxidant protein database30. These antioxidant proteins include vitamins A, C, E and multiple enzymes such as catalase and glutathione peroxidase mostly found in the eukaryotes including plant. Each antioxidant protein has a different function and varied mechanism of action in preventing oxidation and its product. That probably explains why the protein of Moringa’s leaf conferred significantly (P<0.05) higher antioxidation activity compared to the crude extract.
In addition, protein has high potential as a tool in any pharmacological activity since it is the product of central dogma within any biological system including human. Obviously, it is involved in the basic molecular framework of every living organism while the metabolites in the crude extract involved a different pathway. Needless to say protein special ability especially in binding affinity and specificity should not be wasted anymore.
Therefore, extensive studies on antioxidant proteins are still in high demand to unveil their mechanism and full potential especially when oxidative stress are highly related to chronic diseases including diabetes and its complications. Nevertheless, the protein of M. oleifera leaf exhibits promising antioxidant activity worth investigating since this therapeutics plant is highly avail in the environment. Considering its proven ability in ferrous reducing and radical scavenging, sure enough, reports on other therapeutic activity of these plant proteins are going to be drifted hereafter. Simply said, they might be the new drug in the near future provided sufficient researches and investment are made.
Conclusion
The protein extract from commercialised leaf of M. oleifera was able to confer the best antioxidant activity compared to the fresh leaf. This is manifested by the findings of the FRAP and DPPH assays performed on them. Hence, the blooming of this product in the market would benefit its consumers considering of the product’s reputation specifically the antioxidant activity. In addition, the findings of this study should at least trigger further detailed study on the pharmacological activity of the protein extract not only from M. oleifera leaf but also on the processed leaf. This protein could be the new face of the pharmaceuticals industry by replacing current available drugs thus serve the mankind with its special ability.
Acknowledgment
The authors wish to express their gratitude to Universiti Teknologi Malaysia and Zamalah Scholarship award for the platform to conduct this research.
References
- Ramachandran C., Peter K., Gopalakrishnan P. Drumstick (Moringa oleifera): a multipurpose Indian vegetable. Economic botany. 1980; 34(3): 276-283.
CrossRef - Bhargave A., Pandey I., Nama K. S., Pandey M. Moringa oleifera—Sanjana (Horseradish Tree)—A miracle food plant with multipurpose uses in Rajasthan-India—an overview. International Journal of Pure Applied Biosciences. 2015; 3: 237-248.
CrossRef - Dolly J., P. K. R., Amit K., Shikha M., Geeta W. Effect of Moringa oleifera leaves aqueous extract therapy on hyperglycemic rats. Journal of Ethnopharmacology. 2009; 123(3): 392-396.
CrossRef - Kamath N., Swaminathan R., Desai N. Antibacterial activity of Indian medicinal plant-Moringa oleifera against MRSA and Klebsiella Spp.(ESBL) which are commonly isolated bacteria in hospital environments. International Journal of Applied Research. 2016; 2(8): 515-517.
- Torres C. Sanchez J., Osorio H., Sinagawa G., Aguirre A., Gutiérrez D. Moringa oleifera: Phytochemical detection, antioxidants, enzymes and antifugal properties. International Journal of Experimental Botany. 2013; 82: 193-202.
- Jung I. L., Lee J. H., Kang S. C. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncology Letters. 2015; 10(3): 1597-1604.
CrossRef - Anwar F., Latif S., Ashraf M., Gilani A. H. Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy research. 2007; 21(1): 17-25.
CrossRef - Chitra K., Pillai K. S. Antioxidants in health. Indian Journal Physiology Pharmacolology. 2002; 46:1-5.
- Farber J. L. Mechanisms of cell injury by activated oxygen species. Environmental Health Perspectives. 1994; 102(10): 17–24.
CrossRef - Hossain M., Brunton N., Barry-Ryan C., Martin-Diana A., Wilkinson M. Antioxidant Activity of Spice Extracts and Phenolics in Comparison to Synthetic Antioxidants. Rasayan Journal of Chemistry. 2008; 1 (4): 751-756.
- Luqman S., Srivastava S., Kumar R., Maurya A., Chanda D. Experimental Assessment of Moringa oleiferaLeaf and Fruit for Its Antistress, Antioxidant, and Scavenging Potential Using In Vitro and In Vivo Assays. Evidence-Based Complementary and Alternative Medicine. 2012; 1-12.
CrossRef - Sreelatha S., Padma P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleiferaLeaves in Two Stages of Maturity.Plant Foods for Human Nutrition. 2009; 64(4): 303.
CrossRef - Chumark P., Khunawat P., Sanvarinda Y., Phornchirasilp S., Morales N. P., Phivthong-Ngam L., Pongrapeeporn K. U. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera leaves. Journal of Ethnopharmacology. 2008; 116(3): 439-446.
CrossRef - Verma A. R., Vijayakumar M., Mathela, C. S., Rao C. V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera Food and Chemical Toxicology. 2009; 47(9): 2196-2201.
CrossRef - Gopalakrishnan L., Doriya K., Kumar D. S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness. 2016; 5 (2): 49-56.
CrossRef - Congdon R. W., Muth G. W., Splittgerber A. G. The binding interaction of Coomassie blue with proteins. Analytical biochemistry. 1993; 213(2): 407-413.
CrossRef - Spreitzer R. J., Salvucci M. E. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annual review of plant biology. 2002; 53(1): 449-475.
CrossRef - Dipika A. M., Krishna J., Effect of drying on nutritional and functional quality and electrophoretic pattern of soyflour from sprouted soybean (Glycine max). Journal of Food Science Technology. 2010; 47(5): 482–487.
CrossRef - Giami S. Y., Adindu M. N., Hart A. D. et al. Effect of heat processing on in vitro protein digestibility and some chemical properties of African breadfruit (Treculia africanaDecne) seeds.Plant Foods for Human Nutrition. 2001; 56(2): 117-126.
CrossRef - Agoreyo B. O., A. O., Orukpe O. A., O.R., Osaweren Owabor C. N. The Effects of Various Drying Methods on the Nutritional Composition of Musa paradisiaca, Dioscorea rotundata and Colocasia esculenta. Asian Journal of Biochemistry. 2011; 6: 458-464.
CrossRef - Ayegba C., M. O., Obigwa P., Orijajogun J. Effect of Drying Temperature on Nutritional Content of Moringa oleifera World Journal of Food Science and Technology. 2017; 1(3): 93-96.
- Vastag Ž., Popović L., Popović S., Petrović L., Peričin D. Antioxidant and angiotensin-I converting enzyme inhibitory activity in the water-soluble protein extract from Petrovac Sausage (Petrovská Kolbása). Food control. 2010; 21(9): 1298-1302.
CrossRef - Arcan I., Yemenicioglu A. Antioxidant activity of protein extracts from heat-treated or thermally processed chickpeas and white beans. Food Chemistry. 2007; 103(2): 301-312.
CrossRef - Agyei D., Tan K. X., Pan S., Udenigwe C. C., Danquah M. K. Peptides for biopharmaceutical applications. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. 2018; (pp. 231-251). Woodhead Publishing.
CrossRef - Daliri E. B.-M., Oh D. H., Lee B. H. Bioactive Peptides. 2017; 6(5): 32.
CrossRef - Hatanaka T, Uraji M, Fujita A, Kawakami K. Anti-oxidation activities of rice-derived peptides and their inhibitory effects on dipeptidylpeptidase-IV. International Journal of Peptide Research and Therapeutics. 2015;21(4):479-85.
CrossRef - Abdullah F. I., Chua L. S., Rahmat Z., Soontorngun N., Somboon P. Trypsin Hydrolysed Protein Fractions as Radical Scavengers and Anti-bacterial Agents from Ficus deltoidea. International Journal of Peptide Research and Therapeutics. 2018; 24(2): 279-290.
CrossRef - Xie Z., Huang J., Xu X., Jin Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food chemistry. 2008; 111(2): 370-376.
CrossRef - Cumby N., Zhong Y., Naczk M., Shahidi F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chemistry. 2008; 109(1): 144-148.
CrossRef - Feng P., Ding H., Lin H., Chen W. AOD: the antioxidant protein database. Scientific reports. 2017; 7(1): 7449.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





