Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8602
Peer-review started: May 25, 2021
First decision: June 24, 2021
Revised: July 8, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: October 6, 2021
Cerebral infarction is an extremely rare postoperative complication of anterior cervical discectomy and fusion (ACDF), particularly in the delayed setting. We present a case who had a sudden stroke on day 18 after surgery. By sharing our experience with this case, we hope to provide new information about stroke after anterior cervical surgery.
We present the case of a 61-year-old man with more than 20 years of hypertension and 14 years of coronary heart disease who had suffered a stroke 11 years ago. The patient was admitted for a multiple ACDF due to symptoms of cervical spondylotic myelopathy and had a sudden stroke on day 18 after surgery. Imaging findings showed a large-area infarct of his left cerebral hemisphere and thrombosis in his left common carotid artery. With the consent of his family, the thrombus was removed and a vascular stent was implanted through an interventional operation. Forty days later, the patient was transferred to a rehabilitation hospital for further treatment. He had normal consciousness but slurred speech at the 1-year follow-up evaluation. The motor and sensory functions of his hemiplegic limbs partially recovered.
This case illustrated that a postoperative stroke related to anterior cervical surgery may be attributed to prolonged carotid retraction and might have a long silent period. Preventive measures include careful preoperative and postoperative examination for high-risk patients as well as gentle and intermittent retraction of carotid artery sheath during operation.
Core Tip: A postoperative stroke related to anterior cervical surgery is rare and may have a long silent period during which a carotid thrombus had formed at the surgical site. Preventive measures include gentle and intermittent retraction of carotid artery sheath during surgery, careful preoperative risk assessment and grading, and con
- Citation: Jia F, Du CC, Liu XG. Delayed massive cerebral infarction after perioperative period of anterior cervical discectomy and fusion: A case report. World J Clin Cases 2021; 9(28): 8602-8608
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8602.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8602
Anterior cervical discectomy and fusion (ACDF) is an important surgical option in the treatment of cervical degenerative disease, which has a good clinical efficacy and safety with a low incidence of complications[1]. However, since the anterior cervical approach requires intraoperative retraction of carotid artery sheath to expose adequate surgical field, it may affect the hemodynamics of carotid artery and even cause some serious complications in atherosclerotics[2]. In fact, the references from the academic search were limited and insufficient to explain the incidence and timing of cerebral infarction after ACDF. There are only three previous case reports on postoperative cerebral infarction related to cervical spine surgery. Two reports showed cases of stroke occurring immediately after surgery, and the remaining one reported the occurrence at postoperative day 3[3-5]. There has been no report about delayed cerebral infarction occurring after the whole perioperative period of an ACDF.
Herein, we report a case who had a sudden stroke on day 18 after a multilevel ACDF. To the best of our knowledge, this is the first case of massive brain infarction reported after a whole perioperative period. We remind surgeons of this causal relationship segmented by time intervals, discuss the etiology of this rare entity, and report notes on the management.
A 61-year-old man was sent to the emergency room (ER) due to aphasia and right limb dyskinesia when he was doing exercises at home in the early morning.
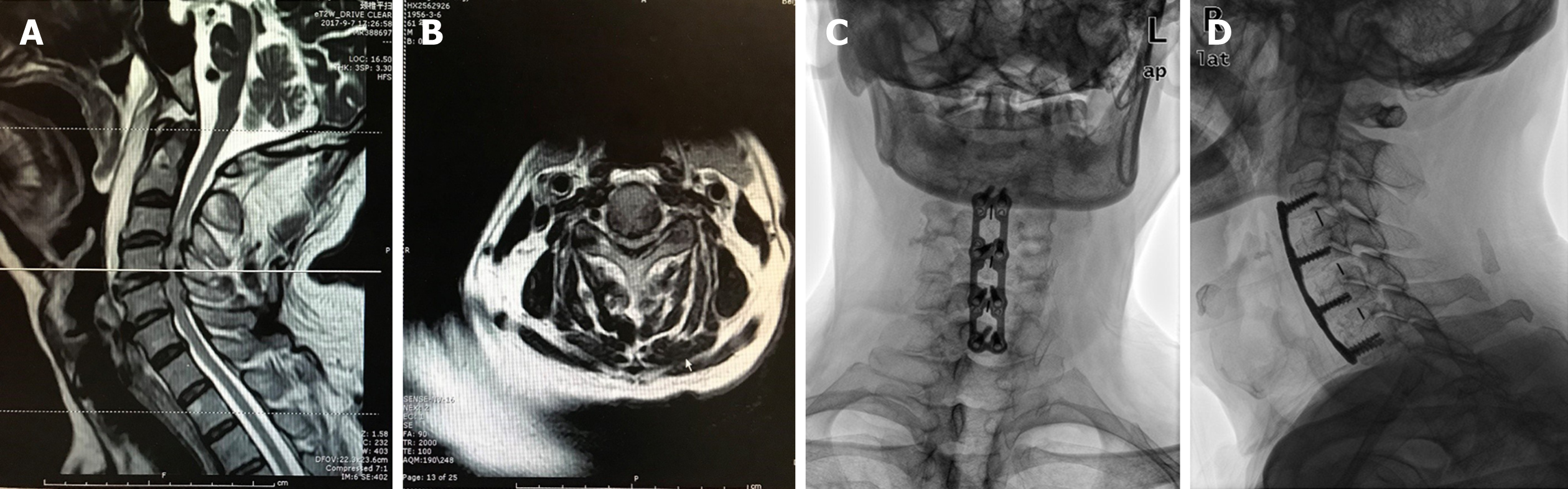
Eighty days ago, the patient sought treatment for a chief complaint of discomfort in the neck and shoulders accompanied with weakness of both upper and lower limbs. The symptoms did not relieve after a full rest. On physical examination, no obvious tenderness but dysfunction of flexion was found in his neck. Pain and hypoesthesia were found in C4, C5, and C6 dermatomes. The muscle strength of scapula stabilization, shoulder abduction, elbow flexion and extension, wrist flexion and extension, and finger flexion was all grade 3 according to the ASIA Grading System. Bilateral Hoffmann's sign was positive and knee tendon reflex was hyperactive. No significant abnormal findings were noted in blood tests. Magnetic resonance imaging (MRI) showed herniated C3-7 discs and hypertrophy of the ligamentum flavum at the C3-6 levels with a wasp-waisted spinal cord (Figure 1A and B). No preoperative vascular examination of the head and neck was performed because it was not a routine item. As a patient with cervical spondylotic myelopathy, he was hospitalized for cervical spine surgery. A multilevel ACDF was performed at the C3-4, C4-5, and C5-6 segments.
During operation, the patient lied in supine position with cervical mild extension, then a left anterior transverse incision at the level of thyroid cartilage was made. After adequate exposure, responsible segments were confirmed with C-arm X-ray images and discectomy was performed with curets and rongeurs. Appropriate cages were implanted into the corresponding disc spaces after sufficient decompression and an anterior cervical plate system was applied. Anterior retraction system was used constantly during the operation. The retractors (self-retaining retractors, WEGO, China) were not always stable at their positions and were adjusted three times. No neurophysiological monitoring techniques were used. Blood pressure, heart rate, and other vital signs were stable during the operation. The operating time was 2 h and the blood loss was about 200 mL.
Postoperatively, the rehabilitation process was satisfactory and the patient was free of complaints about cervical discomfort, but his muscle strength did not recover significantly. The Japanese Orthopaedic Association (JOA) score increased from 8 to 13. Radiographs showed a good cervical curvature and well-positioned internal fixations (Figure 1C and D). No complications developed during his hospital stay. Antihypertensive drugs, aspirin, and low molecular weight heparin were given regularly from the second day after surgery.
At postoperative day 18, when the patient was exercising at home in the early morning, he suddenly developed aphasia, deviated mouth, weakness of the right limbs, urinary incontinence, and unresponsiveness without obvious inducement. There was no headache, dizziness, cough, unconsciousness, or convulsion. Then he was rushed to the emergency room.
The patient had more than 20 years of hypertension while the blood pressure was said to be stable (130-140 mmHg/70-90 mmHg) for years. Besides, he had a 14-year history of coronary heart disease and underwent coronary stent implantation 13 years ago. He took aspirin tablets regularly and was ranked in New York Heart Association (NYHA) class I. The patient had also suffered a cerebral stroke 11 years ago but did not have obvious residual sequelae. Aspirin was stopped after his initial admission.
The patient denied any other relevant personal or family history.
The patient was urgently sent to the ER for evaluation and was found to have a score of 15 on the NIH Stroke Scale. His left internal carotid pulse became weak and a vascular murmur was audible on auscultation. His blood pressure was 160/100 mmHg and speech was slurred. He was conscious but unresponsive. The right nasolabial fold was shallow and the tongue was deviated to the right on extension. The muscle strength of the right limbs decreased significantly, accompanied by superficial hypoesthesia. Hypertonia appeared on his right side. Bilateral Kernig's sign, Brudzinski's sign, and Babinski's sign were all negative.
During the ER treatment period, his counts of white blood cells, red blood cells, and platelets as well as the level of blood glucose were normal. The serum test results were: Homocysteine, 15.65 μmol/L; triglycerides, 2.12 mmol/L; low-density lipoprotein, 4.23 mmol/L; and total cholesterol, 6.42 mmol/L. His D-dimer was 3.92 mg/L and fibrinogen was 4.24 g/L, which were higher than the reference range.
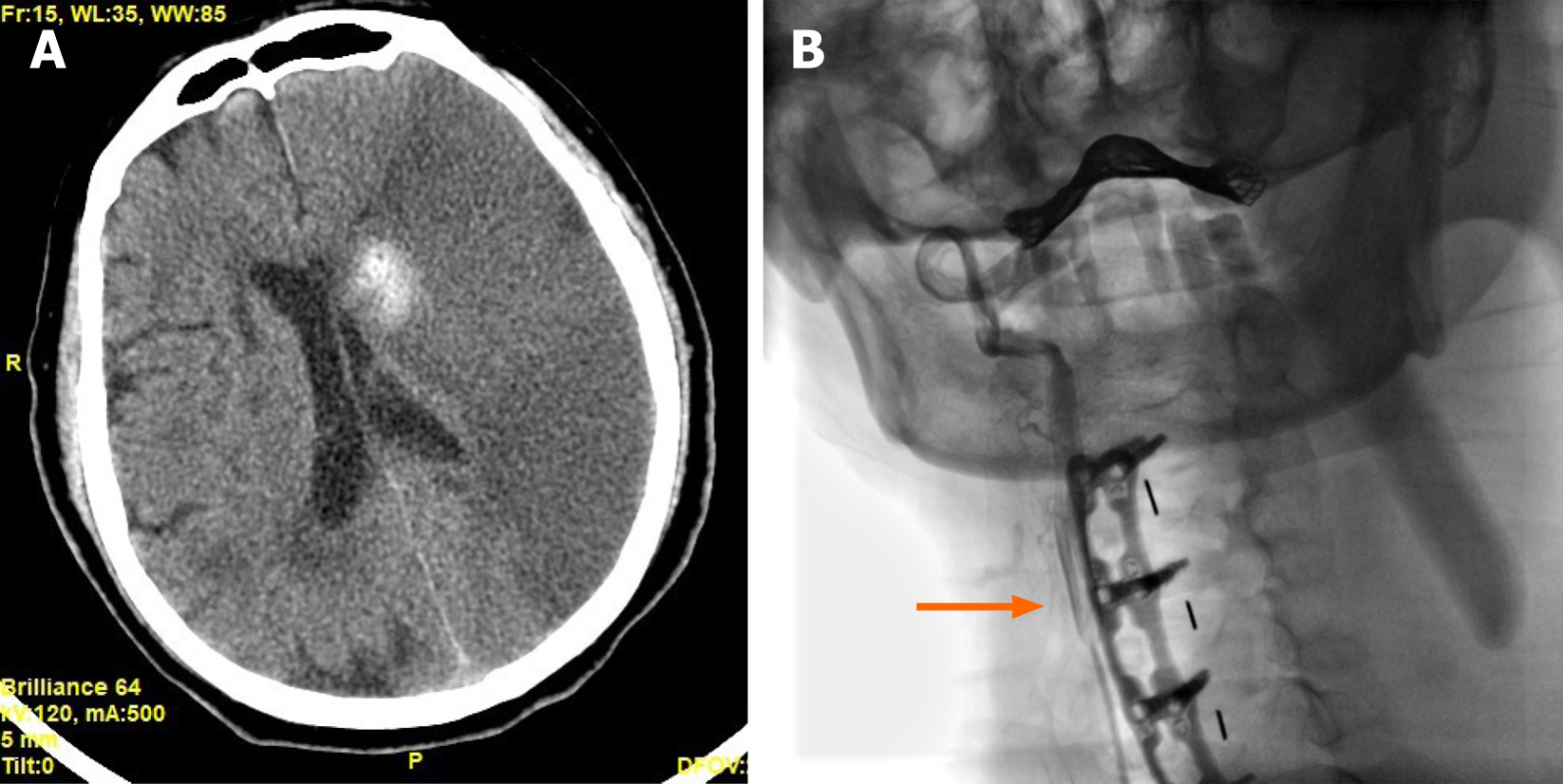
A brain computed tomography (CT) scan showed a diffuse low-density region in his left hemisphere with a periventricular high-density hemorrhage focus (Figure 2A). The patient's condition worsened over time, so a carotid angiography was performed, which showed severe stenosis at the initial segment of his left common carotid artery. The contrast agent could not pass through the stenotic site to the distal end (Figure 2B). The percentage of arteriostenosis was reported at 92% and thrombosis of the left common carotid artery was reported.
Based on the above findings, a diagnosis of common carotid artery thrombosis and a new left hemispheric infarct after cervical spine surgery was established.
With the consent of his family, the thrombus was removed and a vascular stent was implanted with an emergency interventional operation.
Forty days later, the patient was transferred to a rehabilitation hospital for further treatment and exercise. He had normal consciousness but slurred speech at the 1-year follow-up evaluation. The motor and sensory functions of his hemiplegic limbs were partially restored.
We present a case of massive cerebral infarction that occurred 18 d after a multilevel ACDF. It is clear that he had several risk factors for cerebral infarction, including atherosclerotic lesion in the carotid artery, hypertension, coronary heart disease, and previous stroke history[6-9]. On the basis of these factors, discontinuation of aspirin, surgical stress, acute dehydration, and blood pressure fluctuation might collectively precipitate the carotid thrombosis[10,11]. According to the Essen-Stroke-Risk-Score (ESRS) system, the patient should be quantified as median risk (7/9) based on his preoperative status[8,12].
Theoretically, prolonged retraction of the atherosclerotic carotid artery would influence the stability of plaques or even damage the fibrous caps of atheromas, which eventually brought about serious cerebrovascular accidents[13-15]. This is an obvious defect of a long-standing anterior cervical spine surgery. Retraction could also cause changes in carotid artery hemodynamics by reducing cross-sectional area of local vessels but increasing turbulent blood flow[2]. Coupled with perioperative hypercoagulability, all three factors of Virchow were present on one patient and caused the carotid thrombosis[16]. Therefore, we thought that gentle manipulation and inter
According to the search results of Google Scholar, only three case reports of acute cerebral infarction following cervical spine surgery have been published[3-5]. Afana et al[3] deemed that intraoperative surgical manipulation, hypotensive anesthesia, and prolonged neck hyperextension might contribute to their patients’ stroke. Graffeo et al[5] expressed similar sentiments and speculated that the true incidence of cerebral ischemia might be underestimated by current reports, particularly in the delayed setting. Perhaps that was the fact - to the best of our knowledge, this is the first report of cerebral infarction occurring 2 wk after a cervical spine surgery.
It cannot be concluded with certainty that the cerebral infarction was due to mechanical stimulation during the operation, but available evidence tended to prove the point. The angiographic images showed that the thrombus was at the initial segment of the patient’s left common carotid artery, which was exactly the site of intraoperative retraction. It was not the common multiple lacunar infarcts that caused the stroke in this patient but a massive infarct of the left hemisphere. In addition, the case illustrated that a stroke related to anterior cervical spine surgery might have a long silent period during which a carotid thrombus had formed. Patients were at high risk of strokes with few symptoms in this stage and blood pressure fluctuation might play the role of trigger. Besides, the rise of blood pressure could also lead to hemorrhagic strokes with severe symptoms equally[17].
Several studies have shown that aspirin should not be suspended before surgery in patients who have been taking aspirin for a long time, because aspirin has no significant effect on intraoperative and postoperative bleeding[18,19]. Perioperative discontinuation of aspirin may affect coagulation status, thereby increasing the incidence of cardiovascular and cerebrovascular accidents, especially in patients with previous coronary heart diseases or strokes[20]. Perioperative use of anticoagulants such as low molecular weight heparin may also be useful in preventing serious complications[21]. In addition, lipid-lowering drugs can stabilize atheromatous plaques and limit the formation and expansion of thrombosis[22,23]. Preoperative coagulation tests are valuable in evaluating the coagulation status of patients.
Adequate preoperative risk assessment and examination can help identify risk factors for stroke and screen out patients with atherosclerosis. Carotid, transcranial, and cardiac Doppler ultrasound should be performed in high-risk patients before cervical spine surgery to assess the condition of their vessels and the possibility of stroke[24]. If it is clear that there exists severe atherosclerosis unilaterally, an incision from the contralateral side or the feasibility of posterior approach should be considered.
The case has certain warning significance on the postoperative course. The use of antihypertensive agents, lipid-lowering drugs, and anticoagulants should be promptly resumed after surgery[7,21-23]. For patients with definite carotid atherosclerosis, carotid ultrasound can be repeated after surgery to determine the presence of thrombus. For high-risk patients with dizziness, headache, or nausea after surgery, relevant examinations should be performed to exclude cerebral ischemia or infarction. Once it is clear that a patient has carotid thrombosis, aggressive interventions such as thrombectomy or thrombolytic therapy should be used to avoid the occurrence of subsequent stroke with poor clinical outcome[25,26].
This case illustrated that a postoperative stroke related to anterior cervical surgery may be attributed to prolonged carotid retraction and might have a long silent period during which a carotid thrombus had formed at the surgical site. Preventive measures include careful preoperative and postoperative examination for high-risk patients as well as gentle and intermittent retraction of carotid artery sheath during surgery.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: MacCormick AP S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Romano PS, Campa DR, Rainwater JA. Elective cervical discectomy in California: postoperative in-hospital complications and their risk factors. Spine (Phila Pa 1976). 1997;22:2677-2692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Pollard ME, Little PW. Changes in carotid artery blood flow during anterior cervical spine surgery. Spine (Phila Pa 1976). 2002;27:152-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Afana HB, Abuhadrous NMM, Elsharkawy AE. Bithalamic Infarction (Artery of Percheron Occlusion) after Anterior Cervical Discectomy and Fusion. Case Rep Neurol Med. 2019;2019:9438089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Chin KR, Seale J, Butron V, Cumming V. Postoperative cervical haematoma complicated by ipsilateral carotid thrombosis and aphasia after anterior cervical fusion: a case report. Case Rep Med. 2013;2013:590639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Graffeo CS, Puffer RC, Wijdicks EF, Krauss WE. Delayed cerebral infarct following anterior cervical diskectomy and fusion. Surg Neurol Int. 2016;7:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ellekjaer EF, Wyller TB, Sverre JM, Holmen J. Lifestyle factors and risk of cerebral infarction. Stroke. 1992;23:829-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Xiong H, Liu X, Tian X, Pu L, Zhang H, Lu M, Huang W, Zhang YT. A numerical study of the effect of varied blood pressure on the stability of carotid atherosclerotic plaque. Biomed Eng Online. 2014;13:152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Weimar C, Goertler M, Röther J, Ringelstein EB, Darius H, Nabavi DG, Kim IH, Benemann J, Diener HC; SCALA Study Group. Predictive value of the Essen Stroke Risk Score and Ankle Brachial Index in acute ischaemic stroke patients from 85 German stroke units. J Neurol Neurosurg Psychiatry. 2008;79:1339-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Warsz-Wianecka A, Lasek-Bal A, Kazibutowska Z. Cerebral microembolism in patients with segmental left ventricular wall motion abnormalities. Neurol Neurochir Pol. 2014;48:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Vasivej T, Sathirapanya P, Kongkamol C. Incidence and Risk Factors of Perioperative Stroke in Noncardiac, and Nonaortic and Its Major Branches Surgery. J Stroke Cerebrovasc Dis. 2016;25:1172-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Gao Y, Guo Q, Zhang J, Feng Y, Yan B, Su D, Zhu X, Wang G. The underlying risks of circadian blood pressure variation for carotid plaque in treated hypertensive patients with normal blood pressure. Blood Press Monit. 2017;22:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Meng X, Wang Y, Zhao X, Wang C, Li H, Liu L, Zhou Y, Xu J. Validation of the Essen Stroke Risk Score and the Stroke Prognosis Instrument II in Chinese patients. Stroke. 2011;42:3619-3620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Badimon L, Chesebro JH, Badimon JJ. Thrombus formation on ruptured atherosclerotic plaques and rethrombosis on evolving thrombi. Circulation. 1992;86:III74-III85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res. 1999;41:369-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Tripoten MI, Pogorelova OA, Rogoza AN, Balakhonova TV. Comparative investigation of mechanical characteristics of stable and unstable carotid atherosclerotic plaques. Artery Res. 2011;5:191. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Bennett PC, Silverman SH, Gill PS, Lip GY. Peripheral arterial disease and Virchow's triad. Thromb Haemost. 2009;101:1032-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Guo R, Xie Y, Zheng J, Wang Y, Dai Y, Sun Z, Xing L, Zhang X, Sun Y, Zheng L. Short-term blood pressure changes have a more strong impact on stroke and its subtypes than long-term blood pressure changes. Clin Cardiol. 2019;42:925-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Gerstein NS, Schulman PM, Gerstein WH, Petersen TR, Tawil I. Should more patients continue aspirin therapy perioperatively? Ann Surg. 2012;255:811-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Cheng A, Poon MTC, Demetriades AK. Aspirin therapy discontinuation and intraoperative blood loss in spinal surgery: a systematic review. Neurosurg Rev. 2018;41:1029-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Oscarsson A, Gupta A, Fredrikson M, Järhult J, Nyström M, Pettersson E, Darvish B, Krook H, Swahn E, Eintrei C. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. Br J Anaesth. 2010;104:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Kai AM, Vadivelu N, Urman RD, Shukla S, Schonberger R, Banack T. Perioperative Considerations in the Management of Anticoagulation Therapy for Patients Undergoing Surgery. Curr Pain Headache Rep. 2019;23:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Trubelja N, Vaughan C, Coplan NL. The role of statins in preventing stroke. Prev Cardiol. 2005;8:98-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Topcuoglu MA. Transcranial Doppler ultrasound in neurovascular diseases: diagnostic and therapeutic aspects. J Neurochem. 2012;123 Suppl 2:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Brott TG, Haley EC Jr, Levy DE, Barsan W, Broderick J, Sheppard GL, Spilker J, Kongable GL, Massey S, Reed R. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23:632-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 333] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Derex L, Cho TH. Mechanical thrombectomy in acute ischemic stroke. Rev Neurol (Paris). 2017;173:106-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |