Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3000
Peer-review started: February 25, 2019
First decision: April 8, 2020
Revised: May 12, 2020
Accepted: June 20, 2020
Article in press: June 20, 2020
Published online: July 26, 2020
Functional epiphora is a clinical condition which is not due to an anatomic defect. Most studies agree that it involves the action of the orbicularis oculi muscle, particularly its deeper segment (Horner’s muscle), but the exact mechanism is not clear.
To evaluate the orbicularis oculi muscle in functional epiphora patients using electromyography (EMG).
A total of 8 Chinese patients (16 eyes) with functional epiphora were enrolled in this study, and ten volunteers (10 eyes) were included as normal controls. Five epiphora patients (five eyes) with facial palsy served as positive controls. Quantitative EMG was performed in the deeper segment of orbicularis oculi muscle. The average duration of each EMG waveform was measured.
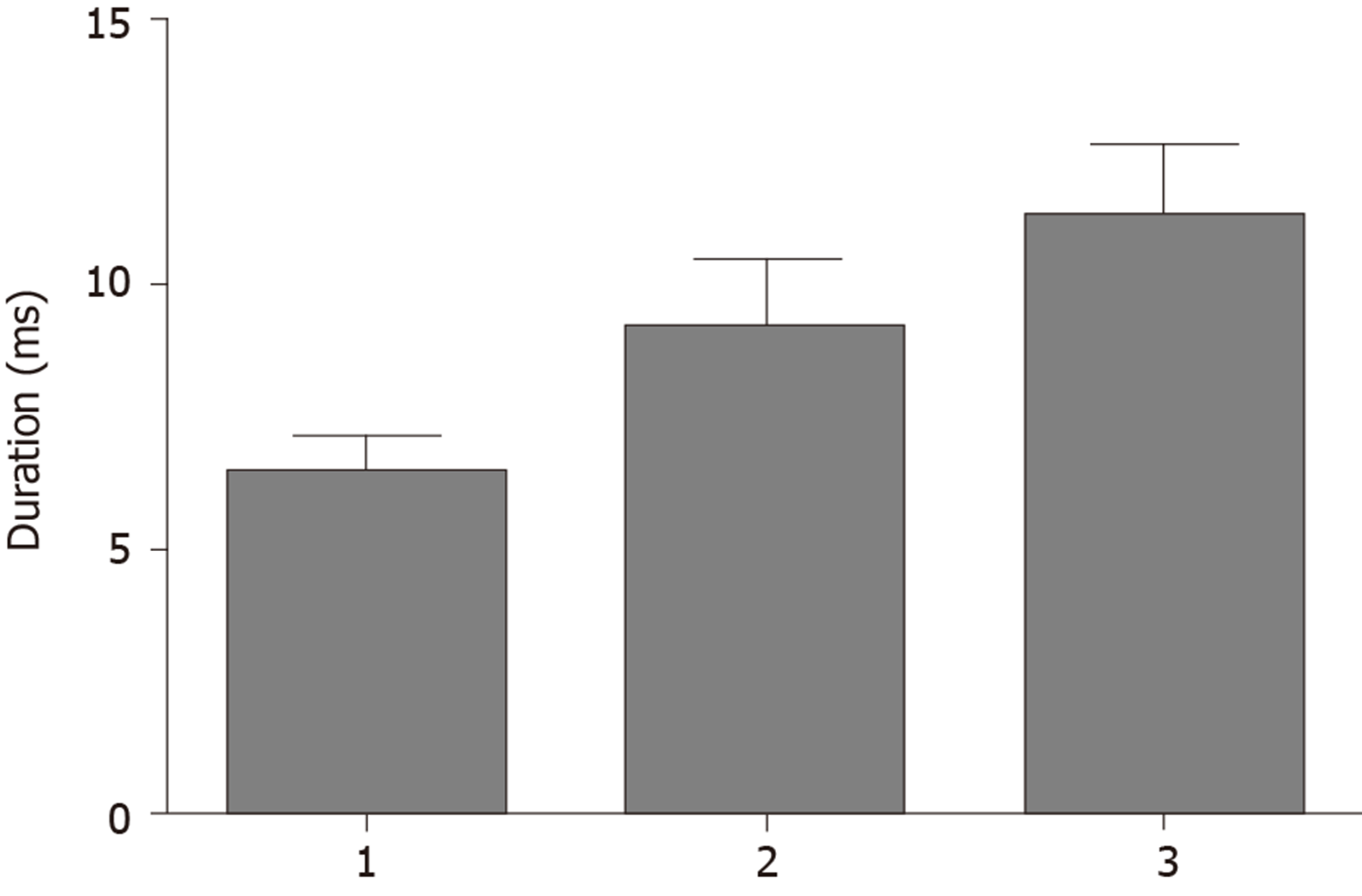
The average duration of EMG waveforms in the normal control group, the functional epiphora group, and the facial palsy group were 6.39 ± 0.73 ms, 9.39 ± 1.32 ms and 11.2 ± 1.42 ms, respectively. The duration of EMG waveforms was significantly longer in the functional epiphora group than in the normal control group (P < 0.05), and shorter than that in the facial palsy group (P < 0.05).
These data indicate the presence of neurogenic orbicularis oculi muscle damage in epiphora patients, which may be the cause of functional epiphora. The etiology of neurogenic damage in the orbicularis oculi muscle requires further investigation.
Core tip: Functional epiphora is a clinical condition which is not due to an anatomic defect, and the cause of functional epiphora is not very clear. In this study, we used electromyography as a valuable tool to evaluate the orbicularis oculi muscle, and the results suggest neurogenic muscle motor neuron disease in functional epiphora patients. This study reveals a possible new approach for the treatment of functional epiphora.
- Citation: Lu H, Liu PD, Yao X, Wang ZF, Gao LF, Wang SP. Diagnostic value of orbicularis oculi muscle electromyography in functional epiphora. World J Clin Cases 2020; 8(14): 3000-3005
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3000.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3000
Epiphora is a bothersome clinical condition, which may require extensive diagnostic efforts. The patient’s history and/or clinical signs, which may include lacrimal hypersecretion, canalicular (presac) obstruction or stenosis, nasolacrimal (postsac) obstruction or stenosis, or even a functional (nonanatomic) defect, which might be due to “lacrimal pump” failure, can provide critical information in identifying this disorder[1]. Functional epiphora is a clinical condition which is not due to an anatomic defect, and the cause of functional epiphora is not very clear[2,3]. Although some controversy exists concerning the exact mechanism of functional epiphora, most studies agree that it involves the action of the orbicularis oculi muscle, particularly its deeper segment (Horner’s muscle), which inserts on the lacrimal sac.
Electromyography (EMG) is a supplement to clinical examination, which can distinguish myopathic from neurogenic muscle wasting and weakness. To determine the etiology of muscle weakness in functional epiphora, we used EMG to evaluate the orbicularis oculi muscle, in order to provide an approach for the diagnosis of functional epiphora.
A total 8 Chinese patients (16 eyes) with functional epiphora were included in this study, 3 males and 5 females aged between 48-68 years, with a mean age of 62.5 years. Five epiphora patients (five eyes) with facial palsy served as positive controls, including 2 males and 3 females aged between 50-70 years, with a mean age of 59.0 years. Patients with chronic lacrimal canaliculitis, previous lacrimal canalicular laceration, congenital absence of lacrimal puncta and canaliculi, or canalicular mass were excluded from the study. Ten volunteers (10 eyes), with a mean age of 62.2 years, without any eye diseases or epiphora symptoms were included in this study as normal controls (Table 1). This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Zibo Central Hospital. All subjects gave written informed consent.
| Normal control group | Functional epiphora group | Facial palsy group | |
| Age, yr | 62.2 ± 7.8 | 62.5 ± 8 | 59.0 ± 6.5 |
| Male/female | 4/6 | 3/5 | 2/3 |
| Subjects (eyes) | 10 (10) | 8 (16) | 5 (5) |
| Duration of EMG (ms) | 6.39 ± 0.73 | 9.14 ± 1.32 | 11.0 ± 1.41 |
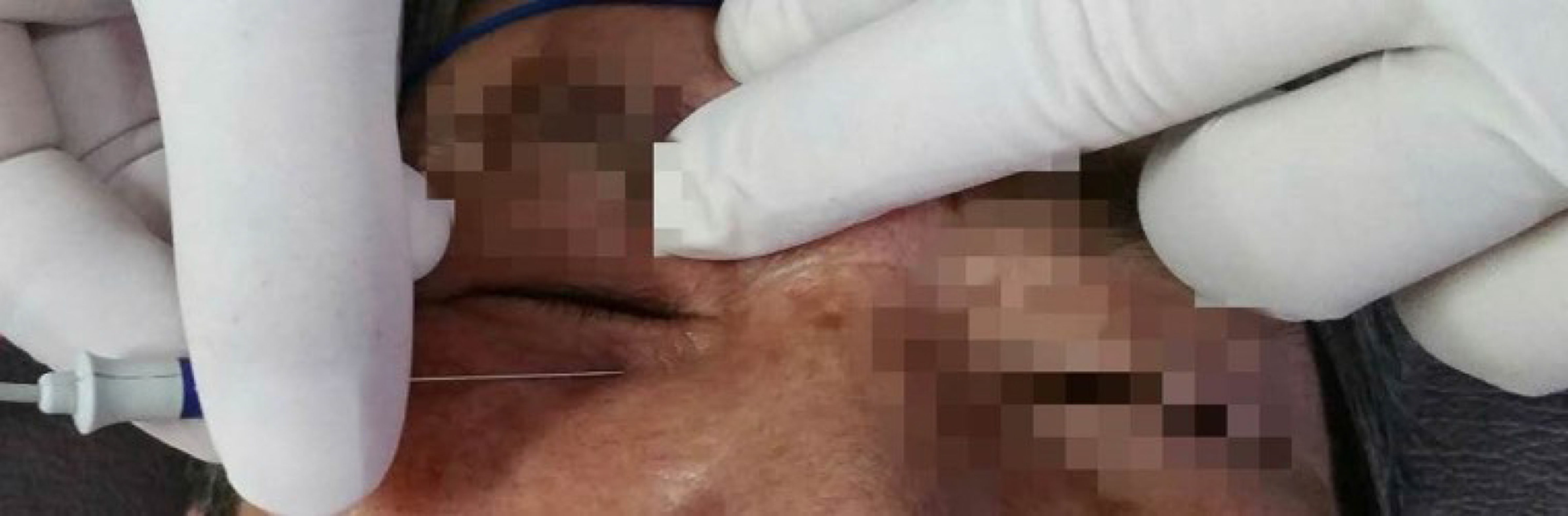
The patency and caliber of the lacrimal puncta was assessed by slit lamp biomicroscopy. Drainage to the ipsilateral nasal cavity was assessed by probing the canalicular system. Quantitative EMG of the orbicularis oculi muscle was performed. EMG response was recorded by a disposable concentric facial EMG needle electrode[4] (25 mm × 0.33 mm, 30G, Alpine Biomed), which was inserted into Horner’s muscle (Figure 1), while the muscle was maintained under slight voluntary contraction. The parameters of the motor unit potential were measured by isolating the discharge of single motor units as achieved by triggering and delaying their display[5-7]. The duration of motor unit potentials was measured. Filter settings were set from 1000 Hz–10000 Hz.
For each eye, the mean duration of the EMG waveform was used in the calculations, and measurements were available from left and right sides. A one-way ANOVA was used to compare the average duration of the EMG waveform in the normal control group, functional epiphora group and the facial palsy group. A P value ≤ 0.05 was considered statistically significant.
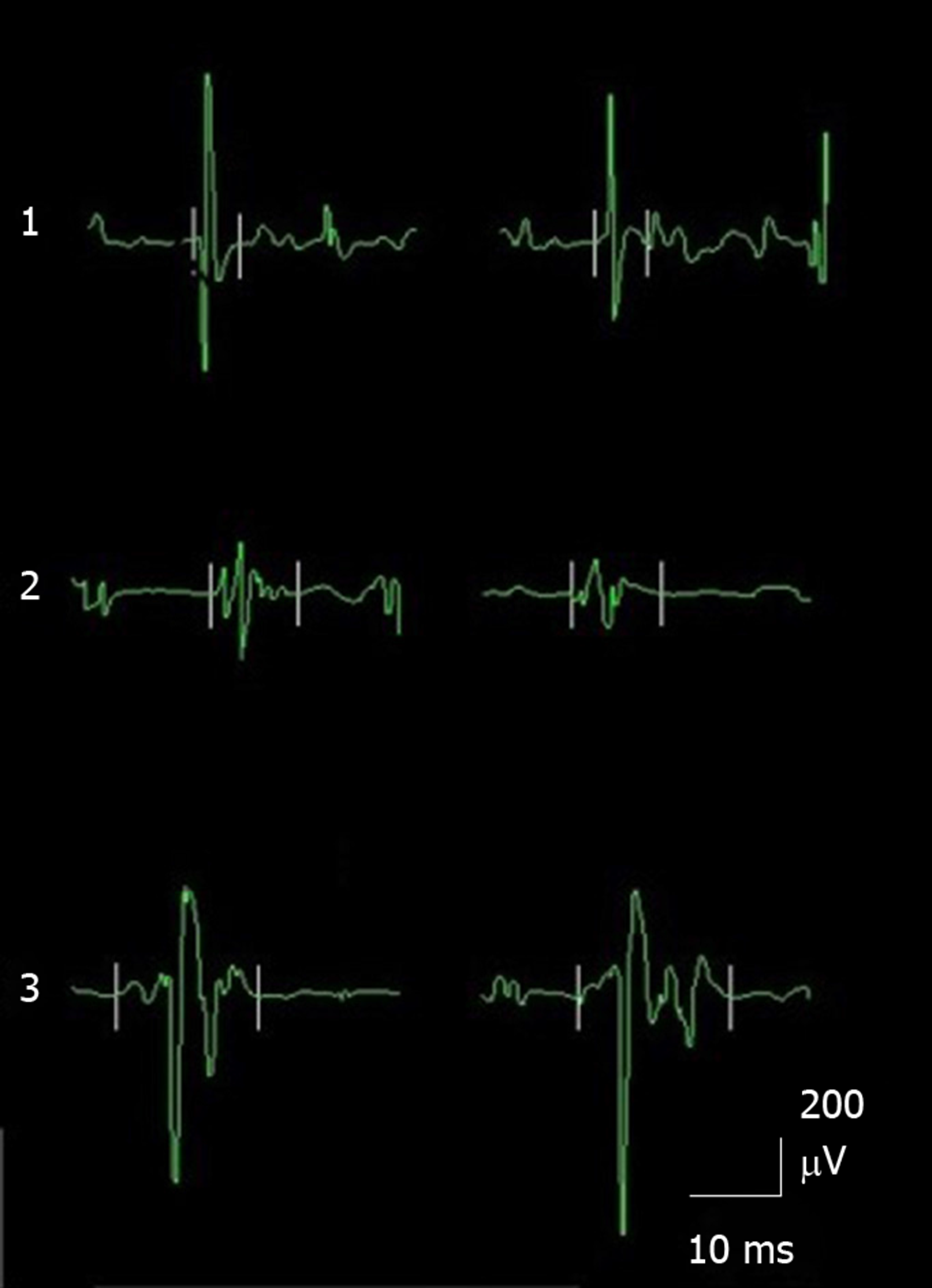
EMG waveforms in the normal control group, the functional epiphora group and the facial palsy group are shown in Figure 2. The average duration of each EMG waveform was measured. The average duration of each action potential was calculated from 7-10 different action potentials. Our data showed that the average duration of the EMG waveform was 6.39 ± 0.73 ms, 9.39 ± 1.32 ms and 11.2 ± 1.42 ms in the control group, the functional epiphora group and the facial palsy group, respectively. The duration of EMG waveforms in the functional epiphora group and facial palsy group were significantly longer than those in the normal control group (P < 0.05), indicating the presence of neurological damage in functional epiphora patients (Figure 3).
Epiphora may present as a watery (usually due to punctual or canalicular causes) or mucous (the so called “sticky eye”) condition[8,9]. Watery epiphora can significantly affect the patient’s quality of life and may be more difficult to treat than mucopurulent discharge[2]. However, the exact reasons underlying epiphora remain unclear. According to Jone’s theory, contraction of Horner’s muscle may cause expansion of the sac and creation of a negative pressure resulting in tear suction[8-10]. Alternatively, the Rosengren Doane theory postulates that the elastic expansion of the lacrimal papillae that occurs upon eyelid opening aspirates tears into the sac and the subsequent contraction of the orbicularis oculi creates a positive pressure gradient that may drive tears along the nasolacrimal duct into the nose[8-10].
The causes of muscle wasting and weakness can be divided into myopathic and neurogenic mechanisms, which can be distinguished by EMG. EMG represents an obligatory tool for assessing myopathic from neurogenic muscle motor neuron disease to demonstrate the widespread denervation and fasciculation required for a comprehensive diagnosis. EMG can detect abnormalities such as chronic denervation or fasciculation, which may not be apparent in clinically normal muscle. Isolating the discharge of single motor units as achieved by triggering and delaying their display, enables parameters of the motor unit potential to be measured. Amplitude and duration were measured, and these motor unit parameters varied with the muscle examined. Chronic re-innervation was associated with long duration motor unit potentials with a normal number of phases. Generally, the amplitude of motor unit potentials was less than 2 mV, and the durations were 10–5 ms with 3–4 phases. Intramuscular sprouting and re-innervation can occur in chronic partial denervation, and the amplitudes might be 10–20 mV and durations might increase to 20–30 ms. In primary muscle disease, only slight motor unit amplitude potentials of short duration were observed; typical amplitude and duration values would be 0.5 mV and 5–10 ms, respectively[11].
A recent study revealed that EMG of the orbicularis oculi muscle is very sensitive in patients with ptosis[12]. In this study, we used EMG to evaluate Horner’s muscle and its relation to functional epiphora. Amplitude and duration were measured, and these motor unit parameters varied with the muscle examined. In general, high amplitude and long duration motor unit potentials with a normal number of phases in EMG suggest chronic re-innervation. Facial palsy is definitely a neurogenic muscle motor neuron disease; therefore, we included facial palsy patients as positive controls in this study. The results demonstrated that the duration of the EMG waveform in the facial palsy patients was significantly longer than that in the normal controls. Consistent with the data from the functional epiphora patients, the duration of the EMG waveform was significantly longer than that in the normal controls, which suggested that chronic denervation in the orbicularis oculi muscle, particularly in the lower segment (Horner’s muscle) may contribute to this condition. It is noteworthy that the extent of increase in the duration of the EMG waveform in the facial palsy patients was more significant as compared to that in the functional epiphora patients, and this may be due to a different degree of neurogenic muscle motor neuron disease.
Thus, longer duration might mean chronic partial denervation, which suggests neurogenic muscle motor neuron disease in the functional epiphora patients, which might help us to treat functional epiphora in another way.
According to previous studies, lacrimal scintigraphy, can identify treatment strategies for functionally acquired epiphora[13,14]. For example, a horizontal shortening of the lower eyelid may be present which can then augment the action of the lacrimal “pump” (as lower eyelid laxity has been associated with decreased lacrimal pump function[13]). However, if the cause of functional epiphora is chronic denervation in the orbicularis oculi muscle, lacrimal scintigraphy may not be good enough to identify treatment strategies for this disease.
In addition, the cross-sectional study method used should be taken into account, the small number of patients included in the study and the lack of a control group for analysis of treatment decision specificity, require further investigation. Our results revealed that EMG of the orbicularis oculi muscle is a valuable tool for identifying treatment approaches for functional epiphora. The etiology of neurogenic damage in the orbicularis oculi muscle requires further investigation.
Functional epiphora is a clinical condition which is not due to an anatomic defect, and the exact causes of epiphora remain unclear. In this study, we used electromyography (EMG) to evaluate the orbicularis oculi muscle, and the results suggested neurogenic muscle motor neuron disease in functional epiphora patients.
Most studies agree that functional epiphora involves the action of the orbicularis oculi muscle, particularly its deeper segment (Horner’s muscle), but the exact mechanism is not clear. In this study, we used EMG to evaluate Horner’s muscle and its relation to functional epiphora, which may provide a new way to evaluate orbicularis oculi muscle-related disease.
The objective of this study was to evaluate the orbicularis oculi muscle in functional epiphora patients using EMG. The data indicated the presence of neurogenic orbicularis oculi muscle damage in epiphora patients, which might be the cause of functional epiphora.
Three groups were included in this study: Functional epiphora, normal controls and facial palsy patients who served as positive controls. Quantitative EMG was performed in the deeper segment of the orbicularis oculi muscle. The average duration of each EMG waveform was measured. A one-way ANOVA was used to compare the average duration of the EMG waveform in the three groups. A P value ≤ 0.05 was considered statistically significant.
The duration of EMG waveforms in the functional epiphora group and facial palsy group were significantly longer than those in the normal control group (P < 0.05), indicating the presence of neurological damage in functional epiphora patients. The small number of patients included in the study and the lack of a control group for analysis of treatment decision specificity, require further investigation.
The cause of functional epiphora is not clear; however, orbicularis oculi muscle weakness might be related to functional epiphora. To determine the etiology of muscle weakness in functional epiphora, we used EMG to evaluate the orbicularis oculi muscle, in order to provide an approach for the diagnosis of functional epiphora. EMG was a valuable tool in evaluating the orbicularis oculi muscle, and the results suggest the presence of neurogenic muscle motor neuron disease in functional epiphora patients, which might help us to treat functional epiphora in another way.
EMG of the orbicularis oculi muscle is a valuable tool for identifying treatment approaches for functional epiphora. The etiology of neurogenic damage in the orbicularis oculi muscle requires further investigation.
The authors would like to thank the members of the Department of Ophthalmology, Zibo Central Hospital.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vaudo G S-Editor: Liu M L-Editor: Webster JR E-Editor: Liu JH
| 1. | Amrith S, Goh PS, Wang SC. Tear flow dynamics in the human nasolacrimal ducts--a pilot study using dynamic magnetic resonance imaging. Graefes Arch Clin Exp Ophthalmol. 2005;243:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Detorakis ET, Drakonaki E, Papadaki E, Pallikaris IG, Tsilimbaris MK. Watery eye following patent external DCR: an MR dacryocystography study. Orbit. 2010;29:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | O'Donnell B, Shah R. Dacryocystorhinostomy for epiphora in the presence of a patent lacrimal system. Clin Exp Ophthalmol. 2001;29:27-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Stålberg EV, Sanders DB. Jitter recordings with concentric needle electrodes. Muscle Nerve. 2009;40:331-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Sanders DB, Stålberg EV. AAEM minimonograph #25: single-fiber electromyography. Muscle Nerve. 1996;19:1069-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Kouyoumdjian JA, Stålberg EV. Reference jitter values for concentric needle electrodes in voluntarily activated extensor digitorum communis and orbicularis oculi muscles. Muscle Nerve. 2008;37:694-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Sarrigiannis PG, Kennett RP, Read S, Farrugia ME. Single-fiber EMG with a concentric needle electrode: validation in myasthenia gravis. Muscle Nerve. 2006;33:61-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Wesley RE. Lacrimal disease. Curr Opin Ophthalmol. 1994;5:78-83. [PubMed] [Cited in This Article: ] |
| 9. | Hurwitz JJ, Cooper PR, McRae DL, Chenoweith DR. The investigation of epiphora. Can J Ophthalmol. 1977;12:196-198. [PubMed] [Cited in This Article: ] |
| 10. | Nesi FA, Waltz KL, Vega J, Nesi FA, Lisman RD, Levine MR. Basic principles of ophthalmic plastic surgery. In: Nesi FA, Lisman RD, Levine MR, editor. Smith's Ophthalmic Plastic and Reconstructive Surgery. Nesi FA, Lisman RD, Levine MR. St. Louis: Mosby, 1998: 81-98. [Cited in This Article: ] |
| 11. | Mills KR. The basics of electromyography. J Neurol Neurosurg Psychiatry. 2005;76 Suppl 2:ii32-ii35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Giannoccaro MP, Di Stasi V, Zanesini C, Donadio V, Avoni P, Liguori R. Sensitivity and specificity of single-fibre EMG in the diagnosis of ocular myasthenia varies accordingly to clinical presentation. J Neurol. 2020;267:739-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Guzek JP, Ching AS, Hoang TA, Dure-Smith P, Llaurado JG, Yau DC, Stephenson CB, Stephenson CM, Elam DA. Clinical and radiologic lacrimal testing in patients with epiphora. Ophthalmology. 1997;104:1875-1881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Maliborski A, Różycki R. Diagnostic imaging of the nasolacrimal drainage system. Part I. Radiological anatomy of lacrimal pathways. Physiology of tear secretion and tear outflow. Med Sci Monit. 2014;20:628-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |