Published online May 6, 2019. doi: 10.12998/wjcc.v7.i9.1066
Peer-review started: January 4, 2019
First decision: January 26, 2019
Revised: February 21, 2019
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: May 6, 2019
Mitochondrial diseases are a heterogenous group of multisystemic disorders caused by genetic mutations affecting mitochondrial oxidation function. Brain involvement is commonly found in most cases but rarely as the unique clinical manifestation. Since the knowledge of its clinical manifestation combined with genetic testing is important for preventing misdiagnosis and delay in treatment, we report here how we diagnosed and managed a very unusual case of mitochondrial encephalopathy.
We report a 52-year-old woman with recurrent stroke-like episodes carrying the m.10158T>C mutation in the MT-ND3 gene, which is also responsible for fatal infant-onset Leigh syndrome. Despite the common mutation, the present case featured a distinct clinical and neuroimaging manifestation from Leigh syndrome. This patient presented with sudden onset of right-sided hemiparesis and hemilateral sensory disturbance accompanied by a left temporal cluster-like headache and later developed epilepsy during hospitalization, with no other signs suggestive of myopathy, lactate acidosis, or other systemic symptoms. Brain magnetic resonance imaging revealed variable lesions involving multiple cortical and subcortical regions. Furthermore, a negative genetic test obtained from peripheral blood delayed the diagnosis of mitochondrial disease, which was eventually established through second-generation DNA sequencing using biopsied muscle.
Based on this report, we suggest that clinicians pursue proper genetic testing for patients when the clinical phenotype is suggestive of mitochondrial diseases.
Core tip: An adult-onset stroke-like episode combined with a distinctive magnetic resonance imaging finding serves as a key diagnostic feature indicating mitochondrial disease. A negative peripheral blood genetic test does not necessarily exclude mitochondrial disease, and muscle biopsy is necessary, even with a lack of muscular symptoms.
- Citation: Fu XL, Zhou XX, Shi Z, Zheng WC. Adult-onset mitochondrial encephalopathy in association with the MT-ND3 T10158C mutation exhibits unique characteristics: A case report. World J Clin Cases 2019; 7(9): 1066-1072
- URL: https://www.wjgnet.com/2307-8960/full/v7/i9/1066.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i9.1066
Diagnosis of mitochondrial disease is challenging. Symptoms such as a stroke-like episode, lactic acidosis, deafness, diabetes mellitus, short stature, and myopathy, often with a family history showing maternal inheritance, are the most common chara-cteristics of a mitochondrial disease. Additionally, a stroke-like episode often occurs in childhood or adolescence. Nonetheless, a diagnosis of mitochondrial diseases in patients with adult onset is often delayed due to a lack of family history, atypical manifestations, and limited resources for functional, pathologic, and/or genetic testing in routine clinical practice[1]. We report an adult-onset patient with mitochondrial encephalopathy carrying the m.10158T >C mutation in the MT-ND3 gene. The encephalopathy manifested as isolated recurrent stroke-like episodes without clinical myopathy or other organ dysfunction. In this paper, we focus on how we diagnosed this case and the distinct feature of the brain magnetic resonance imaging (MRI), which is important for preventing misdiagnosis and delay in treatment. Additionally, we summarize the clinical implications of such cases with this mutation.
A 52-year-old female presented with a sudden onset of right-sided numbness and weakness that was accompanied by a left temporal cluster-like headache. No fever or prodromal infection was found at disease onset.
The family and personal history was unremarkable.
On physical examination, the height and weight of the patient were 154 cm and 56 kg, respectively. Vital signs were normal, as were heart, lung and abdominal exami-nations. Neurological examination showed intact mental status, with normal speech and comprehension. Mild 4/5 right-sided hemiparesis was present with normal tone in both the arm and leg, though no other focal neurological deficits were found. After admission, she complained of discomfort and tingling in the right leg, after which a generalized tonic-clonic seizure for 3 min occurred before it was stopped by a bolus of intravenous diazepam.
Laboratory tests, including D-dimer, lactic acid, and serum autoantibody levels, as well as thyroid function and tumor markers indicated no apparent abnormalities. Glucose tolerance and lactic acid movement tolerance tests were normal. A lumbar puncture was performed, and her open intracranial pressure was 180 mm H2O. Cerebrospinal fluid (CSF) testing showed that cell counts and protein, glucose, chloride, monoclonal antibody, adenosine deaminase, and lactate dehydrogenas levels were within normal ranges.
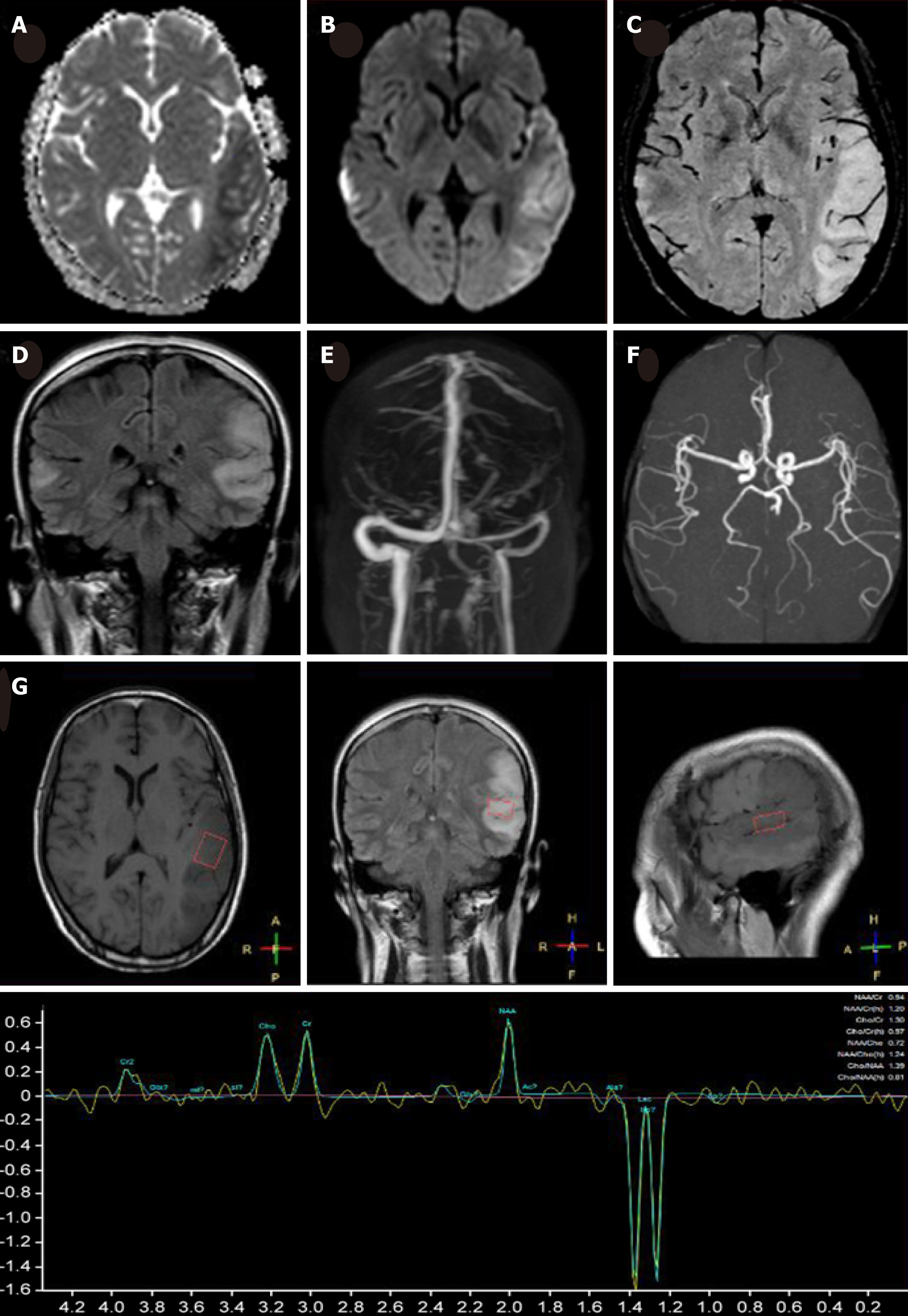
MRI demonstrated a lamellar left parietal lobe lesion predominantly involving the cortex, with hyperintensity on both diffusion-weighted imaging and fluid-attenuated inversion recovery (Figure 1). The apparent diffusion coefficient map revealed a preserved, isointense signal. No abnormalities were found by susceptibility weighted imaging or magnetic resonance angiography and venography (Figure 1). Due to the stroke-like onset pattern and MRI features, further thrombophilia screening was performed and showed decreased protein S activity. A diagnosis of cortical venous thrombosis was first proposed. Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) was also considered but temporarily excluded because of the incomplete manifestation and lack of genetic evidence. Anticoagulation therapy was initiated, and follow-up was performed to maintain the international normalized ratio (INR) within the target range.
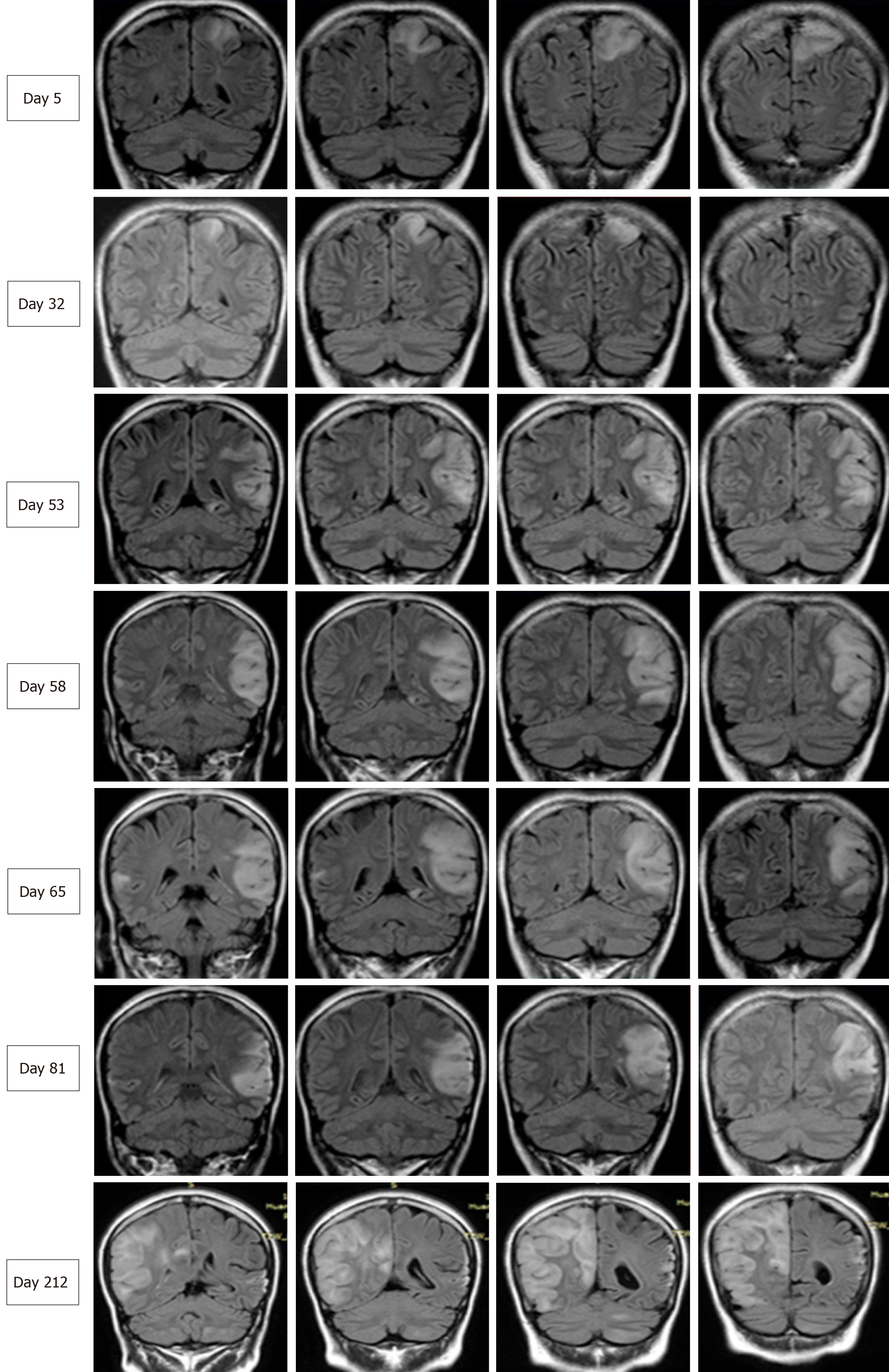
Two months later, the patient was readmitted for subacute cognitive impairment. She was unable to identify and communicate with family members; she also had difficulty understanding questions or instructions and instead responded by repeating the word "nothing". During hospitalization, a secondary generalized seizure occurred, initially with eyes gazing to the right and then convulsion developing, which lasted for approximately 10 s before self-alleviation. Neurological examination suggested transcortical sensory aphasia, with fully covered limb strength. Blood tests and CSF examination were normal; INR was 2.21. On repeated MRI, new lesions were identified in the left temporal lobe and were also detected 10 days later in the right temporal lobe on radiological follow-up (Figures 1 and 2). Although the MRI signal characteristics are consistent with the initial findings, the original lesion in the left parietal lobe had been alleviated, with cortical atrophy. We further conducted magnetic resonance spectroscopy (MRS), which revealed markedly elevated lactate (Lac) concentrations in the regions of interest in the left temporal lesion (Figure 1). Mitochondrial encephalopathy was diagnosed, and genetic testing using peripheral blood was performed. However, DNA testing for frequent MELAS and myoclonic epilepsy with ragged red fibers syndrome mutations were negative. Because of the lack of symptoms of muscle weakness or pain, the patient declined our suggestion of performing a muscle biopsy. Anticoagulation therapy was terminated, and levetiracetam (1000 mg/d) was administered.
At 3 mo after her second admission, the patient was experiencing involuntary movement in her left limbs, with repetitive flexion/extension. An MRI scan showed a hyperintense signal abnormality in the right parietal lobe (Figure 2). Brachial biceps biopsy was performed. Histopathology revealed no abnormalities, and no necrotic or regenerating fibers were observed; ragged-red fibers and intense succinate dehydrogenase activity were not detected. Nonetheless, complete sequencing of mitochondrial DNA samples extracted from the biopsied muscle revealed a heteroplasmic m.10158T>C mutation, with a heteroplasmy level of 69.6%, in the mitochondrial complex I subunit gene MT-ND3. In contrast, this mutation was not found in her peripheral blood cells.
The diagnosis was MELAS syndrome harboring the m.10158T>C mutation.
We administered levetiracetam (1000 mg/d) and oxcarbazepine (400 mg/d) to control epilepsy. Q10 and L-arginine were also administered.
Over the next 6 mo, the present patient did not manifest any further stroke-like episodes.
We herein report a case of adult-onset mitochondrial encephalopathy, whereby the patient was admitted three times for similar stroke-like episodes before the definitive diagnosis was confirmed. Despite highly suggestive MRI features of MELAS syndrome, the diagnosis of mitochondrial encephalopathy was delayed due to the following reasons: (1) Lack of family history showing maternal inheritance; (2) Isolated symptoms of CNS impairment, without myopathy, lactic acidosis, or other systemic disorders; and (3) Negative mitochondrial genetic testing using peripheral blood. Although muscle biopsy revealed no abnormality, genetic testing using the biopsied muscle demonstrated a 69.6% heterozygous T10158C mutation in the MT-ND3 gene.
Although MELAS is not uncommon in clinical practice, its diagnosis may be challenging because the clinical symptoms are highly variable and genetic findings may be absent. Our patient presented with a headache, seizure, and acute focal lesion observed by MRI, which fit the diagnostic criteria of supportive MELAS by Yatsuga et al[2]. Although her level of lactic acid in blood was normal, the presence of an inverted lactate peak was detected in the temporal cortex by MRS, which is a useful tool to detect metabolic dysfunction in the brain[3]. Additionally, despite no obvious myopathy, the biopsied muscle showed a 69.6% heteroplasmic mutation, T10158C, in the MT-ND3 gene.
The MT-ND3 protein is a structural component of multimeric enzyme complex I of the mitochondrial respiratory chain, which drives ATP generation in mitochondria[4]. The MT-ND3 T10158C mutation, which is located in the coding region of the loop domain of the ND3 subunit protein, has been identified in infants and pediatric patients with Leigh syndrome or Leigh-like disease[5]. However, the manifestation of late-onset stroke-like encephalopathy similar to MELAS in our patient harboring the MT-ND3 T10158C mutation highlights the complicated genotype-phenotype relationship in mitochondrial diseases. In a literature review, we found four similar cases of MT-ND3 T10158C mutation (in muscle)[6,7], and all of these patients presented with adult-onset, isolated involvement of the central nervous system. Moreover, MRI revealed similar features among these five patients, with lesions predominately in the posterior cortex of the supratentorial region; in contrast, MRI typically shows involvement of the basal ganglia, brainstem, and cerebellum in Leigh syndrome. Thus, adult-onset patients harboring the MT-ND3 T10158C mutation may exhibit unique clinical features. Although no myopathy symptoms were observed in any of these cases and genetic testing using peripheral blood was negative, muscle specimens should be obtained to establish a definitive diagnosis. This may be explained by heteroplasmic mtDNA mutations in various tissues, in which case, the mutation load in muscles is higher than that in blood[8].
Adult-onset mitochondrial encephalopathy in the presence of the MT-ND3 T10158C mutation shows unique clinical characteristics. Despite a lack of lactic acidosis, myopathy, or other clinical implications, the diagnosis of MELAS needs to be con-sidered for adult-onset patients with a stroke-like episode of encephalopathy. Due to the heteroplasmic mutation load, a negative genetic test obtained when using peripheral blood does not necessarily exclude the diagnosis of mitochondrial disease, and next-generation DNA sequencing using biopsied muscle should be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Razek AAKA S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Grier J, Hirano M, Karaa A, Shepard E, Thompson JLP. Diagnostic odyssey of patients with mitochondrial disease: Results of a survey. Neurol Genet. 2018;4:e230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, Kakuma T, Koga Y; Taro Matsuoka for MELAS Study Group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. 2012;1820:619-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | El-Mewafy ZMH, Razek AAKA, El-Eshmawy MM, El-Eneen NRA, El-Biaomy AAB. Magnetic resonance spectroscopy of the frontal region in patients with metabolic syndrome: correlation with anthropometric measurement. Pol J Radiol. 2018;83:e215-e219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Galkin A, Meyer B, Wittig I, Karas M, Schägger H, Vinogradov A, Brandt U. Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I. J Biol Chem. 2008;283:20907-20913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Bugiani M, Invernizzi F, Alberio S, Briem E, Lamantea E, Carrara F, Moroni I, Farina L, Spada M, Donati MA, Uziel G, Zeviani M. Clinical and molecular findings in children with complex I deficiency. Biochim Biophys Acta. 2004;1659:136-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Mukai M, Nagata E, Mizuma A, Yamano M, Sugaya K, Nishino I, Goto YI, Takizawa S. Adult-onset Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis, and Stroke (MELAS)-like Encephalopathy Diagnosed Based on the Complete Sequencing of Mitochondrial DNA Extracted from Biopsied Muscle without any Myopathic Changes. Intern Med. 2017;56:95-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Mezuki S, Fukuda K, Matsushita T, Fukushima Y, Matsuo R, Goto YI, Yasukawa T, Uchiumi T, Kang D, Kitazono T, Ago T. Isolated and repeated stroke-like episodes in a middle-aged man with a mitochondrial ND3 T10158C mutation: a case report. BMC Neurol. 2017;17:217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Rossignol R, Faustin B, Rocher C, Malgat M, Mazat JP, Letellier T. Mitochondrial threshold effects. Biochem J. 2003;370:751-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 538] [Article Influence: 25.6] [Reference Citation Analysis (0)] |