Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.2953
Peer-review started: June 22, 2019
First decision: August 1, 2019
Revised: August 20, 2019
Accepted: August 26, 2019
Article in press: August 26, 2019
Published online: October 6, 2019
Hyperthyroidism in pregnancy may pose a great threat to maternal and fetal health. The risk of hyperthyroid heart disease (HHD), even heart failure, is significantly elevated in pregnant women.
To investigate the clinical characteristics, prognosis, and therapy of HHD in pregnant women.
We searched the patient registry data at West China Second University Hospital of Sichuan University in Chengdu, China, following the approval by the Ethics Committee. We retrospectively analyzed the clinical characteristics of pregnant women diagnosed with HHD. The medical records of women with HHD during pregnancy from January 2012 to December 2017 were obtained from the electronic medical records system. All the included patients were followed in outpatient clinics and by telephone interviews until October 2018.
A total of 155 patients were diagnosed with thyrotoxicosis, of whom six were diagnosed with HHD. Three of them had regular antenatal care. Two patients were complicated with acute heart failure attacks, and one of them had a stillbirth. Both of these patients had a long history of Graves’ disease with poor treatment compliance. Treatments of precipitating factors such as the control of infection could relieve the symptoms and prolong gestation for a better prognosis. Hyperthyroid heart failure could be controlled with aggressive diuretics and management of the coexisting complications. Intense monitoring and timely anti-heart failure treatment were crucial in patients with severe cardiac damage. Our findings indicated the importance of regular antenatal care and treatment adherence in patients with hyperthyroidism.
The timely and accurate diagnosis of HHD and the implementation of effective management are important for a better prognosis in pregnant women with HHD. Improvement in patients’ awareness of thyrotoxicosis is needed.
Core tip: Hyperthyroidism may pose a great threat to maternal and fetal health and may increase maternal and fetal mortality. Approximately 85% of hyperthyroidism cases result from Graves’ disease. We retrospectively analyzed a case series of pregnant patients with hyperthyroid heart disease from a central referral hospital in Southwest China. The significance of regular monitoring and the application of anti-thyroid treatment are implied. The control of precipitating factors is important for the management of heart failure.
- Citation: Shan D, Bai Y, Chen QH, Wu YX, Chen Q, Hu YY. Hyperthyroid heart disease in pregnancy: Retrospective analysis of a case series and review of the literature. World J Clin Cases 2019; 7(19): 2953-2962
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/2953.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.2953
Hyperthyroidism may pose great a threat to maternal and fetal health and may increase maternal and fetal mortality[1-3]. The prevalence of hyperthyroidism in pregnant women is about 0.05% to 3.0%, with approximately 85% of these cases resulting from Graves’ disease (GD)[1,4]. Cardiac function is greatly influenced by thyroid hormone levels, and an overload of thyroid hormones could can affect systematic hemodynamics and lead to high-output heart failure and cardiomyopathy[5]. Heart failure is rare when the basal high-output state is compensated in non-pregnant patients. However, the risk for developing to heart failure is elevated in pregnant patients with untreated thyrotoxicosis due to the increased cardiac burden during pregnancy[6,7], particularly in patients with precipitating factors such as preeclampsia, anaemia, and infection, in which cases, the hemodynamic workload is even more increased.
The management of hyperthyroidism in pregnancy includes the application of anti-thyroid drugs (ATDs) and regular monitoring of thyroid function[1,8]. The goal of treatment is to control thyrotoxicosis as early as possible to prevent further adverse pregnancy outcomes. Studies performed in developed countries implied the importance of immediate treatment and regular monitoring[7,9,10]. However, in China, there are economically undeveloped areas where patients rarely have antenatal care. Ignorance of doctors’ suggestions is common in patients of lower socioeconomic levels. The implementation of the “two-child” policy might make this problem even worse. The aim of our retrospective study was to review clinical cases of pregnant patients with hyperthyroid heart disease (HHD) enrolled at our hospital in order to evaluate maternal and fetal outcomes and provide a supplement to the literature.
We searched the patient registry data at West China Second University Hospital of Sichuan University in Chengdu, China, following the approval by the Ethics Committee. Our hospital is the central referral center in Southwest China. All the patients’ written consent to use their clinical data for research purpose was obtained at admission. The medical records of women with HHD during pregnancy from January 2012 to December 2017 were obtained from the electronic medical records system in our hospital.
For patients who received antenatal care in our hospital, thyroid function was a routine test, and the suggestions from an endocrinologist were followed in cases of abnormal thyroid function. For patients transferred to our hospital, a thyroid function test was performed immediately after admission. For patients who had diffuse toxic goiter, Graves’ ophthalmopathy or other variable findings, such as tachycardia, palpitations, hyperreflexia, and irritability, a thyroid function test was performed immediately upon admission, and consultation comments from an endocrinologist were taken. The diagnosis of GD was established according to the American Thyroid Association and Endocrine Society Guidelines for patients with biochemical evidence of overt hyperthyroidism and clinical symptoms for GD[1,8]. HHD was confirmed by cardiologists in patients who presented with any type of arrhythmia, thyrotoxic cardiomyopathy, or heart failure. Cardiac structure and function were assessed using electrocardiography, 24-h dynamic electrocardiogram, chest radiography, and thoracic ultrasound. Patients with underlying cardiac abnormalities, such as congenital heart defects or valvular heart disease, were excluded. Patients with critical illnesses such as renal failure, liver failure, cancer, or malignant hematologic diseases were also excluded. All the included patients were followed in outpatient clinics and by telephone interviews until October 2018.
In our hospital, the clinical treatment of pregnant women with HHD included propylthiouracil (PTU) or methimazole (MMI) for anti-hyperthyroidism therapy according to the consultation opinion from an endocrinologist. Propranolol was used to control arrhythmia. In patients with heart failure, furosemide was used for pulmonary edema and cardiac glycosides were applied. Non-invasive positive pressure ventilation therapy (NIPPV) and continuous renal replacement therapy (CRRT) were used in some critical patients. Preeclampsia was treated with magnesium sulfate, and blood transfusion was administered in patients with anemia. Infection was treated with wide-spectrum antibiotics. Regular monitoring of the fetal condition was applied. Dexamethasone injection and magnesium sulfate were used to improve fetal lung development in patients at a risk of preterm birth.
During the 5-year study period, 69389 women delivered at our hospital. A total of 155 patients were diagnosed with hyperthyroidism, and six patients were diagnosed with HHD, with the New York Heart Association classification of cardiac function ranging from III to IV. Two of the patients with HHD were further complicated with acute heart failure attacks. Both of these two patients had been previously diagnosed with GD but used ATDs irregularly without thyroid function monitoring or preconception counselling. Only three patients had regular antenatal care. Another patient never had antenatal care during pregnancy. Thyroid function tests revealed that the total thyroxine (T4) and triiodothyronine (T3) levels ranged from 176.8 nmol/L to 390.0 nmol/L and 2.99 nmol/L to 7.67 nmol/L, respectively. The plasma levels of thyroid globulin antibody (TGAb) and thyroid peroxidase antibody (TPOAb) were very high in these six patients. The follow-up of these patients showed good outcomes. Tables 1 and 2 present the clinical features of the six patients.
| Demographic | Vital signs at admission | Cardiac evaluation at admission | Complications and aggravating factors | NYHA |
| 26 yr G5P1+3 (31 + 4 wk) | T 36.5 °C, HR 153 bpm, BP 141/78 mmHg, R 32 breaths/min, SpO2 85%; Vaginal examination revealed blood-stained mucus and a dilated cervix of 1 cm | Echocardiography: Enlarged left atrium and ventricle (LA 31 mm, LV 46 mm); EF 25%. Thoracic ultrasound: Bilateral pleural effusion, left 3.5 cm right 3.0 cm | Scarred uterus, stillbirth | IV |
| 24 yr G2P0+1 (35 + 1 wk) | T 36.6 °C, HR 110 bpm, BP 168/98 mmHg, R 30 breaths/min, SpO2 89% | Echocardiography: Enlarged left atrium and ventricle (LA 44 mm, LV 58 mm); moderate mitral regurgitation (Vmax 4.0 m/S); EF 53%. Chest radiography: Cardiomegaly and mild bilateral pleural effusion; patchy consolidation in low lobes of lung of both sides; Cardiothoracic ratio 0.61 | Preeclampsia, pulmonary infection, anaemia | IV |
| 30 yr G2P1 (34 + 4 wk) | T 37.4 °C, HR 130 bpm, BP126/80 mmHg, R 35 breaths/min, SpO2 94% | Echocardiography: Enlarged left atrium and ventricle (LA 39 mm, LV 51 mm); grossly mitral regurgitation (Vmax 5.3 m/S); EF 50%. Chest radiography: Cardiomegaly and mild bilateral pleural effusion; cardiothoracic ratio 0.59. Thoracic ultrasound: Bilateral pleural effusion, left 0.8 cm right 2.9 cm | Anaemia, pulmonary infection | IV |
| 29 yr G3P1+1 (30 + 4w) | T 38.3 °C, HR 130 bpm, BP 138/80 mmHg, R 40 breaths/min, SpO2 88% | Echocardiography: Enlarged left atrium (LA 40 mm); moderate mitral regurgitation (Vmax 5.6m/S); EF 62% | Chorioamnionitis? | III |
| 24 G4P1+2 (38 + 6 wk) | T 36.3 °C, HR 100 bpm, BP 113/70 mmHg, R 25 breaths/min, SpO2 95% | Echocardiography: Normal, EF 59%. 24 hours dynamic electrocardiogram: normal | Scarred uterus, pulmonary infection | III |
| 27 yr G1P0 (38 + 6 wk) | T 36.5 °C, HR 80 bpm, BP 102/60 mmHg, R 25 breaths/min, SpO2 97% | Echocardiography: Enlarged left atrium and ventricle (LA 39 mm, LV 62 mm); EF 37%. 24-hour dynamic electrocardiogram: Premature ventricular contractions | Pulmonary infection | III |
| Patient | Clinical course |
| 1 | No antenatal care. Previous history: Diagnosed with Graves’s disease at age 12 and treated with antithyroid treatment till 16. Lower extremity edema for 3 mo, and palpitations and heat intolerance for 3 wk. No fetal movement for 1 day, and regular uterine contraction for 12 h. Altered level of consciousness after admission, oxygen was immediately administered via a facemask, recovered to consciousness. Artificial rupture of membranes and Willett-Martel scalp flap forceps were used to deliver the fetus. Transferred to ICU after delivery. 5 times of acute heart failure attacks in 3 d after delivery. Except for normal approach, CRRT and NIPPV were applied. Blood plasma and albumin were given. After reaching a stable situation, she was discharged at day 7 at patients’ request. Follow-up: Conservative treatment in local hospital for a month after delivery. I131 treatment one year after delivery, recovered well. Normal thyroid function at follow-up |
| 2 | Irregular antenatal care in local hospital. Previous history: Diagnosed with Graves’s disease at 14, irregular treatment with ATD therapy 2 yr before, stopped treatment during pregnancy. Cough with phlegm for 10 d, and generalized edema, dyspnea, and palpitations for 3 d. Treatments included PTU, propranolol, and antibiotics. Magnesium sulfate was used for pree-clampsia, and blood transfusion was applied for anaemia. Onset of labour at her 35 + 5 wk. Presented with symptoms and signs of heart failure during the second stage of labour, digoxin injection, furosemide, and oxygen mask were administered immediately to arrest heart failure, and continuous intra-venous pumping of nitroglycerin was used to control high blood pressure. Gave birth vaginally with the assistance of vacuum to a female baby weigh-ing 2010 g, Apgar 7-8-9. Admitted to NICU. Discharged at day 9. Follow-up: Chest radiography revealed small patchy consolidation in low lobes of right lung two days after delivery. Chest radiography returned to normal 6 wk after delivery, but thyroid function test still showed high levels of T3 and T4. Used ATDs regularly and had thyroid function test every half year. Normal thyroid function and stopped ATD treatment 5 yr after delivery |
| 3 | Irregular antenatal care in local hospital. Never been diagnosed as hyperthyroidism. Previous history: Accepted blood transfusion 1 mo before in local hospital. Generalized edema for 1 month, and cough, orthopnea, and dyspnea for 4 d. Treatments included PTU, propranolol, antibiotics, furose-mide, magnesium, and dexamethasone. Gave birth vaginally at her 36 wk to a female baby weighing 2080 g, Apgar 9-10-10. Discharged at day 12. Follow-up: Chest radiography returned to normal 6 wk after delivery. Used ATDs for one year after delivery, then thyroid function test returned to normal |
| 4 | Regular antenatal care in local hospital. Diagnosed with Graves’s disease at local hospital at 14 wk. PTU was applied after diagnosis. Previous history: Treated with PTU, propranolol, magnesium sulfate, and antibiotics at local hospital for a week. Lower extremity edema for 1 mo, and anxiety and palpitations for 2 wk. Enlargement of left atrium and atrial fibrillation was found in local hospital without appropriate treatment. Increased vaginal discharge and suspected premature rupture of membrane for 1 wk, and dyspnea and orthopnea for 2 d. Treatments included increased dose of PTU, propranolol, more potent antibiotics, frusemide, and magnesium sulfate. Regular monitoring included blood white cell count, C-reactive protein, procalcitonin, and vaginal secretion culture. Gave birth vaginally to a male baby at her 33 + 2 wk weighing 2220 g, Apgar 10-10-10. Admitted to NICU. Discharged at day 20. Follow-up: Chest radiography returned to normal 6 wk after delivery. Used ATDs and had thyroid function test regularly, and used MMI 5 mg per day |
| 5 | Regular antenatal care. Previous history: Diagnosed with Graves’s disease for loss of weight and exophthalmos 2 mo before pregnancy, then regularly treated with ATDs. Cough with phlegm, dyspnea, and palpitations at 34 wk. Enlarged left atrium was found. Hospitalization treatments included antibiotics, PTU, propranolol, and magnesium sulfate for 10 d. Cough and moderate palpitations for 2 d. Treatments included MMI and antibiotics. A female baby weighing 3360 g was delivered by caesarean section at her 39 + 4 wk, Apgar 10-10-10. Discharged at day 8. Lost to follow-up |
| 6 | Regular antenatal care at local hospital. Previous history: Diagnosed with Graves’s disease at age 22, RAI therapy at 24, and continuous treatment with ATD. Moderate palpitations for 3 mo, cough and heart intolerance for 3 d, and rupture of membrane for 6 h. Treatment included PTU. A female baby weighing 3860 g was delivered by caesarean section at her 39 wk, Apgar 10-10-10. Discharged at day 6. Follow-up: Echocardiography showed only enlarged left ventricle and normal EF six days after delivery. Normal chest radiography and normal thyroid function 6 wk after delivery |
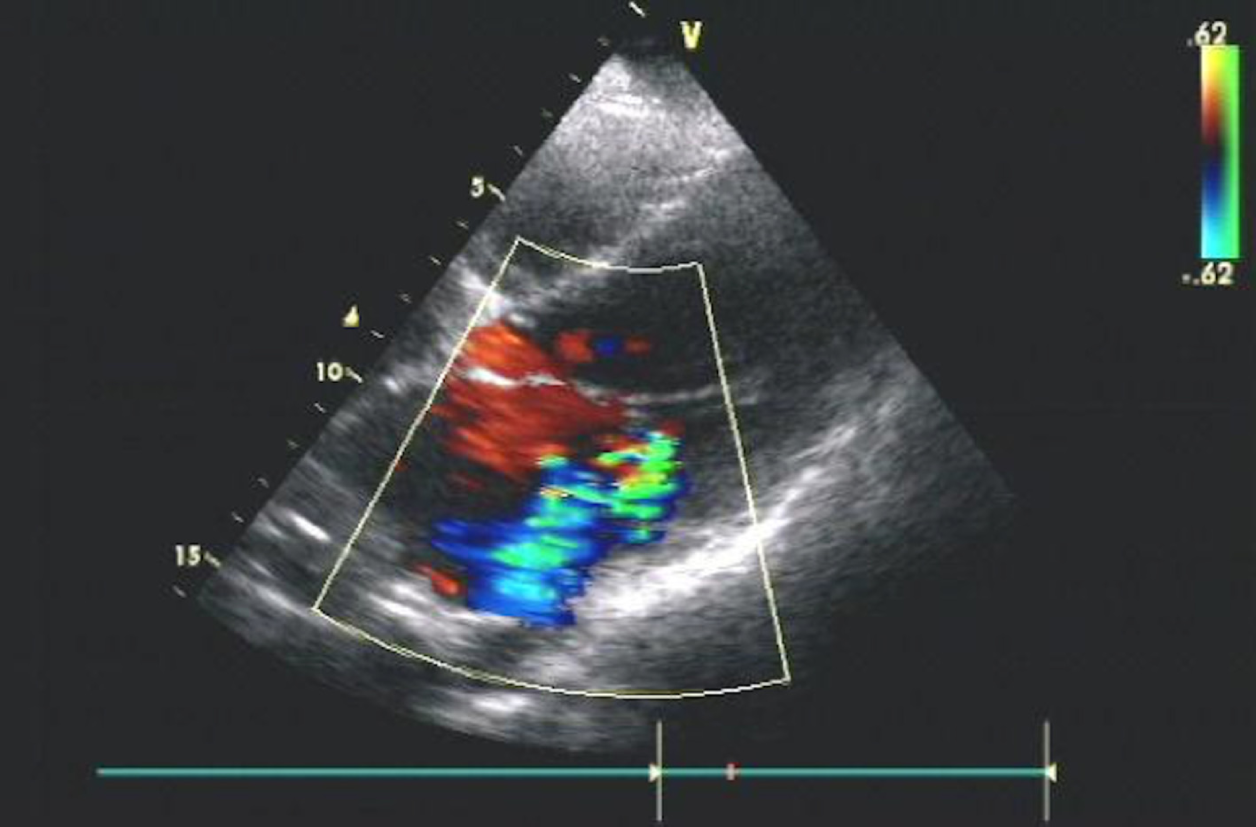
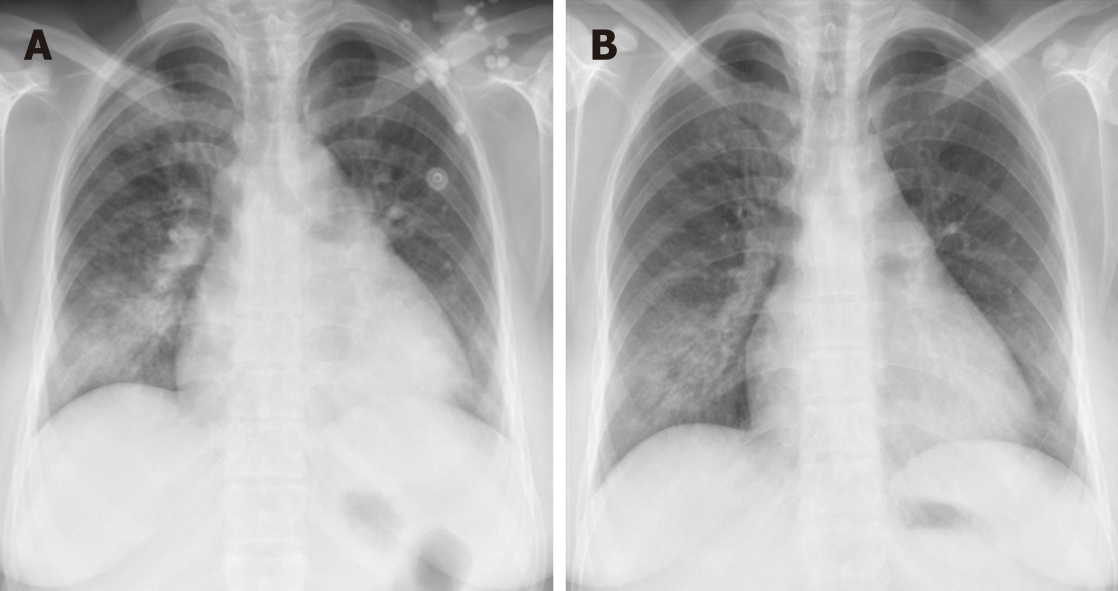
The number of gestational weeks at delivery ranged from 31 + 4 wk to 39 + 4 wk. Two patients experienced acute heart failure attacks in the second stage of labour. Vacuum and forceps were implemented in these patients to shorten the labour time. One patient who had a stillbirth had several heart attacks even after delivery. NIPPV and CRRT were used. The other patient responded well to aggressive diuretics and cardiac glycosides. This was confirmed by chest radiography and echocardiography. Figure 1 presents the echocardiography, and Figure 2 presents the comparison of chest X-rays. Two patients were enrolled with manifestations of heavy cardiac burden, but after receiving appropriate treatments for GD and precipitating factors, both patients responded well and prolonged their pregnancy. Two healthy preterm infants were born vaginally at 33 wk and 36 wk, respectively. These two patients did not present any symptoms of heart failure during labour. Table 3 shows the changes in thyroid hormone levels in these two patients. Another two patients who had regular antenatal care chose an elective caesarean section delivery, and two healthy full-term infants were born. In these six patients, the identified precipitating factors included anaemia, preeclampsia, pulmonary infection, and suspected chorioamnionitis. All of the patients’ symptoms were relieved to a certain extent after the precipitating factors were treated.
| Initial | 1 wk | 2 wk | Reference range | ||
| Patient 3 | Before delivery | ||||
| TSH (mIU/L) | < 0.003 | 0.003 | 0.006 | 0.55-4.78 | |
| Total T3 (nmol/L) | 7.67 | 2.83 | 2.54 | 0.92-3.7 | |
| Total T4 (nmol/L) | > 387 | > 387 | 295.1 | 58.1-173 | |
| Free T3 (pmol/L) | 16.7 | 6.31 | 4.53 | 3.5-6.5 | |
| Free T4 (pmol/L) | 61.91 | 42.75 | 35.43 | 11.5-22.7 | |
| TGAb (IU/mL) | > 500 | > 500 | > 500 | < 60 | |
| TPOAb (IU/mL) | > 1300 | > 1300 | > 1300 | < 60 | |
| Initial | 1 wk | 2 wk | 3 wk | ||
| Patient 4 | Before delivery | ||||
| TSH (mIU/L) | 0.008 | 0.008 | 0.01 | 0.006 | 0.55-4.78 |
| Total T3 (nmol/L) | 4.01 | 1.95 | 1.60 | 1.72 | 0.92-3.7 |
| Total T4 (nmol/L) | 194 | 122.80 | 76.7 | 69.4 | 58.1-173 |
| Free T3 (pmol/L) | 9.15 | 4.97 | 4.03 | 3.57 | 3.5-6.5 |
| Free T4 (pmol/L) | 23.79 | 13.79 | 8.58 | 7.75 | 11.5-22.7 |
| TGAb (IU/mL) | > 500 | > 500 | > 500 | > 500 | < 60 |
| TPOAb (IU/mL) | > 1300 | > 1300 | > 1300 | > 1300 | < 60 |
The prevalence of thyrotoxicosis in pregnant women in our study was 0.22%, which is lower than that in other provinces of China[11,12]. Six of 155 patients were diagnosed with HHD, and the incidence of heart disease in the pregnancies with thyrotoxicosis was 3.87%. The clinical course and progression were different in these six patients. First, we found that the pregnancy outcomes were favorable in patients with regular antenatal care, regular ATD treatment, and continuous monitoring of maternal and fetal conditions. However, in patients without regular antenatal care who deliberately disregarded their doctors’ suggestions, the risk of adverse pregnancy outcomes was highly elevated. Particularly in patients with a long history of GD but with poor medical compliance, HHD could pose a great threat to maternal and fetal health. Second, most patients had precipitating factors, including infection, anemia, or preeclampsia. Treatment of these precipitating factors could relieve the symptoms. Prolongation of gestation for a better prognosis for the fetus is possible when the maternal cardiac burden is reduced. Considering the existing cardiac load, magnesium sulfate but not ritodrine should be applied for the prevention of preterm birth in these patients. Third, hyperthyroid heart failure responded quickly to diuretic treatment and the control of the coexistent complications. In patients with severe cardiac damage, intense monitoring of vital signs and timely treatment against acute cardiac failure attacks are still very important even after delivery.
The prognosis and pregnancy outcomes in our reported patients were similar to those reported in the literature. Sheffiled et al reported 13 patients with thyrotoxicosis, 6 of whom had heart failure. Similar decompensation reasons, such as hemorrhage and sepsis, were revealed in their study[7]. Mannisto et al analyzed singleton pregnancies from 2002 to 2008 in a single center and found that hyperthyroidism significantly elevated risks for preeclampsia, preterm birth, and ICU admission[9]. Thirty patients from a prospective study in Ukraine reported relief of cardiac symptoms following ATD treatment and improvement in the health-related quality of life[10]. The importance of good compliance and regular use of ATDs were also implicated in other studies[13,14].
Patients with HHD may have various manifestations, and focusing on the cardiac symptoms and ignoring thyroid hormones test results may lead to delays in diagnosis. One of the six patients had never been diagnosed with GD before, the first hospital that was visited provided blood transfusion for anemia and antibiotics, and these therapies partially relieved some symptoms. In pregnant patients, the correct diagnosis and administration of effective management of hyperthyroidism might be even more difficult. Both pregnancy and hyperthyroidism could manifest with symptoms of hyperdynamic circulation and hypermetabolism. The diagnosis of hyperthyroidism should be suspected if the women presents with specific symptoms like exophthalmos or symptoms of hyperdynamic circulation and hypermetabolism. A systematic blood screening including thyroid function test is also very important and sometimes might compensate if the clinical symptoms are ignored.
All six patients had symptoms including dyspnea, orthopnea, or chest tightness at 30-34 wk when the aggravation of cardiac burden was most obvious due to the expansion of blood volume. Duration and inadequate control are the main factors impacting prognosis[2,8,15]. Four of our patients were diagnosed with GD before enrollment, but only two of them adhered to ATD treatment. Differences in treatment compliance determined their differences in pregnancy outcomes. Two patients in our study had acute heart failure attacks. In a retrospective study conducted in America including 13 patients with HHD, most of the patients were also at the third trimester with precipitating factors[7]. As the heart is the main target organ of thyroid hormone, hyperthyroidism can lead to several adverse changes in cardiac function[5,16]. Increased catecholamine function and activation of the renin-angiotensin-aldosterone system also contribute[3,5]. The main manifestations of thyrotoxic cardiomyopathy include left ventricular hypertrophy, disturbances in heart rhythm, atrial fibrillation, enlargement of the heart chambers, and heart failure[5,16]. These symptoms were all present in our two patients. Hyperthyroid heart failure is considered as “curable” after the achievement of a euthyroid state, and the patient usually responds quickly to aggressive diuretics[7,14]. However, in the patient who had a stillbirth, the cardiac function was damaged irreversibly due to the long-term exposure to the thyrotoxicosis status. NIPPV and CRRT were applied. Effective treatment by a multidisciplinary medical team including obstetricians, paediatricians, cardiologists, endocrinologists, anaesthetists, and intensive care unit doctors is needed.
ATD is the first line treatment for hyperthyroidism in both nonpregnant and pregnant patients. All of our patients received ATDs, and most of the cardiovascular symptoms were relieved. A Ukrainian study revealed the beneficial effects of ATD treatment on health-related quality of life (HRQoL)[10]. Pregnancy and breastfeeding are the absolute contraindications to radioiodine therapy (RAI), and those who accepted RAI treatment should use contraception methods for at least for 6 mo[8]. One of our patients had RAI before pregnancy. She had good compliance after RAI and counselled with multidisciplinary doctors before her pregnancy. Patients who received thyroidectomy, similar to RAI, should also avoid pregnancy for 6 mo to obtain a euthyroid state and improve the successful conception rate[3,8].
ATD drugs, including MMI and PTU, are commonly used among pregnant patients with thyrotoxicosis. Both PTU and MMI can cross the placenta at comparable rates and influence fetal thyroid function. PTU has been preferred over MMI in the first trimester due to the considerably lower risk for birth defects[1,8]. But a recent study showed that there was no difference in rates of congenital malformations between women treated with MMI and those treated with PTU[17]. Reports have indicated that the rates of birth defects in PTU and MMI are similar to the overall birth defect rate in America and even lower than the birth defect rate in China[18-20]. One of our patients with regular antenatal care received PTU until the second trimester, and then she was treated with MMI. A follow-up interview of our patients showed a preference for MMI over PTU after delivery. Thyroid stimulating hormone receptor antibodies can also enter the infant’s circulation and cause fetal GD. None of the newborns of our patients were found to have GD or congenital defects. Considering the high relapse rate of GD in the puerperium period, suggestions of continuous ATD treatment were given to our patients. In all the patients diagnosed with HHD in our hospital, appointments for thyroid and heart function assessments in the puerperium period were made before discharge from the hospital, which ensured a better prognosis even after delivery.
This study addresses a significant gap in the literature on the outcomes of HHD in pregnant women in Southwest China. An extensive search of the medical record system and good follow-up ensured that detailed clinical data of importance, including blood test results and imaging examinations, were analyzed. These patients were enrolled from a single medical center and, therefore, ascertained the same diagnostic criteria, treatment, and prenatal monitoring. However, this study has limitations that should be noted. Medical records rather than direct patient interviews were used, and some information provided in the follow-up may have led to recall bias. As this was a summary of a single-center experience, the results from this study may not be generalizable to economically developed cities.
Our findings indicated the importance of regular antenatal care and treatment adherence in patients with hyperthyroidism. The importance of a multidisciplinary medical team to ensure timely and effective management in pregnant women with HHD and better prognoses for both mothers and infants was also implied. Making the correct diagnosis of HHD in pregnant women is difficult yet essential for the administration of effective management. Precipitating factors should be effectively eliminated to reduce the cardiac burden. Patients with GD should adhere to an anti-thyroid regimen as soon as possible after the diagnosis is confirmed. Consultation before pregnancy is needed. Improvement in antenatal care and enhanced awareness of thyrotoxicosis in pregnant women are needed, especially in rural and urban areas.
In summary, we have reported six pregnant patients with HHD who delivered in our hospital. Detailed medical records were used to retrieve the patients’ clinical data. Differences in their clinical courses and compliance rates determined the differences in their pregnancy outcomes. The follow-up study showed good results. This case series report demonstrates some important issues for the clinical approach for women with HHD. With the widespread implementation of the “second child” policy in China, the number of women with thyroid disease and the number of pregnant women with HHD are both increasing, and it is important for obstetric doctors in China to emphasize prenatal care in these patients.
In pregnant women, the prevalence of hyperthyroidism is approximately 0.05% to 3.0%, and most of these cases are caused by Graves’ disease. If the clinical management of hyperthyroidism is not provided in time, it could lead to hyperthyroid heart disease (HHD), which is a serious condition in pregnant women that has life-threatening risks.
The investigation of the clinical characteristics of pregnant patients with HHD can improve our understanding of this serious complication. Given that pregnancy can increase the hemodynamic workload and is a challenge to the cardiac function, pregnant women suffer more risks from HHD than the general population.
The main objective of this study was to review the clinical courses of pregnant patients with HHD enrolled in a central referral hospital in Southwest China in order to provide evidence for better management of this serious complication.
The electronic medical records system was searched for the collection of patient data. The medical records of women with HHD during pregnancy from January 2012 to December 2017 were obtained. Follow-up was completed by outpatient clinic visits and telephone interviews.
During the study period, a total of nearly 70000 women delivered at our hospital. Six patients were diagnosed with HHD. Two patients with a long history of Graves’ disease with poor treatment compliance suffered acute heart failure attacks, one of whom had a stillbirth. Three patients had regular antenatal care, and the prognosis was comparatively better. HHD could be controlled after the application of anti-thyroid drugs and aggressive diuretics and the management of the coexisting complications. Intense monitoring and timely anti-heart failure treatment were important for the prognosis in patients with severe cardiac damage.
We present the clinical courses of six patients with HHD, and differences in their compliance lead to their different pregnancy outcomes. Making a correct diagnosis and providing a timely intervention are crucial. The regular application of anti-thyroid drugs before and during pregnancy can prevent the development of HHD. Treatments of precipitating factor can relieve the cardiac burden to some extent.
Due to the retrospective nature of the study and the small sample size, it is difficult to draw reliable conclusions. However, our findings indicate the importance of good treatment compliance, regular antenatal care, and timely treatment in pregnant patients with HHD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El Amrousy D S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Liu JH
| 1. | De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543-2565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 857] [Cited by in F6Publishing: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 2. | King JR, Lachica R, Lee RH, Montoro M, Mestman J. Diagnosis and Management of Hyperthyroidism in Pregnancy: A Review. Obstet Gynecol Surv. 2016;71:675-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Nguyen CT, Sasso EB, Barton L, Mestman JH. Graves' hyperthyroidism in pregnancy: a clinical review. Clin Diabetes Endocrinol. 2018;4:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Azizi F, Amouzegar A. Management of hyperthyroidism during pregnancy and lactation. Eur J Endocrinol. 2011;164:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Osuna PM, Udovcic M, Sharma MD. Hyperthyroidism and the Heart. Methodist Debakey Cardiovasc J. 2017;13:60-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Kriplani A, Buckshee K, Bhargava VL, Takkar D, Ammini AC. Maternal and perinatal outcome in thyrotoxicosis complicating pregnancy. Eur J Obstet Gynecol Reprod Biol. 1994;54:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Sheffield JS, Cunningham FG. Thyrotoxicosis and heart failure that complicate pregnancy. Am J Obstet Gynecol. 2004;190:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26:1343-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1305] [Cited by in F6Publishing: 1254] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 9. | Männistö T, Mendola P, Grewal J, Xie Y, Chen Z, Laughon SK. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J Clin Endocrinol Metab. 2013;98:2725-2733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Tsymbaliuk I, Unukovych D, Shvets N, Dinets A. Cardiovascular complications secondary to Graves' disease: a prospective study from Ukraine. PLoS One. 2015;10:e0122388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96:3234-3241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, Zhou J, Mao J, Yu X, Li J, Chen Y, Xue H, Fan C, Wang H, Zhang H, Li C, Zhou W, Gao B, Shang T, Zhou J, Ding B, Ma Y, Wu Y, Xu H, Liu W. The prevalence of thyroid disorders during early pregnancy in China: the benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. 2011;164:263-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Aggarawal N, Suri V, Singla R, Chopra S, Sikka P, Shah VN, Bhansali A. Pregnancy outcome in hyperthyroidism: a case control study. Gynecol Obstet Invest. 2014;77:94-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Riaz K, Forker AD, Isley WL, Hamburg MS, McCullough PA. Hyperthyroidism: a "curable" cause of congestive heart failure--three case reports and a review of the literature. Congest Heart Fail. 2003;9:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Krassas G, Karras SN, Pontikides N. Thyroid diseases during pregnancy: a number of important issues. Hormones (Athens). 2015;14:59-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet. 2016;388:906-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 420] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 17. | Gianetti E, Russo L, Orlandi F, Chiovato L, Giusti M, Benvenga S, Moleti M, Vermiglio F, Macchia PE, Vitale M, Regalbuto C, Centanni M, Martino E, Vitti P, Tonacchera M. Pregnancy outcome in women treated with methimazole or propylthiouracil during pregnancy. J Endocrinol Invest. 2015;38:977-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Yoshihara A, Noh J, Yamaguchi T, Ohye H, Sato S, Sekiya K, Kosuga Y, Suzuki M, Matsumoto M, Kunii Y, Watanabe N, Mukasa K, Ito K, Ito K. Treatment of graves' disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J Clin Endocrinol Metab. 2012;97:2396-2403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Yu M, Ping Z, Zhang S, He Y, Dong R, Guo X. The survey of birth defects rate based on birth registration system. Chin Med J (Engl). 2015;128:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Kirby RS. The prevalence of selected major birth defects in the United States. Semin Perinatol. 2017;41:338-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |