Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3311
Peer-review started: February 2, 2023
First decision: February 17, 2023
Revised: February 23, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: May 16, 2023
Neisseria mucosa is a gram negative diplococcus belonging to the genus Neisseria found commonly in the upper respiratory tract. It is typically a commensal organism when it is parasitic on oral and nasal mucosa. To our knowledge, it does not cause disease in healthy individuals with normal immunity, but can be pathogenic in those with impaired immune function or change in bacterial colonization site. Neisseria mucosa has been reported to cause bacterial meningitis, conjunctivitis, pneumonia, endocarditis, peritonitis and urethritis. However, peritoneal dialysis-related peritonitis caused by Neisseria mucosa is extremely rare in clinical practice, which has not previously been reported in China.
A 55-year-old female presented to the nephrology clinic with upper abdominal pain without apparent cause, accompanied by nausea, vomiting and diarrhea for two days. The patient had a history of Stage 5 chronic kidney disease for five years, combined with renal hypertension and renal anemia, and was treated with peritoneal dialysis for renal replacement therapy. The patient was subsequently diagnosed with peritoneal dialysis-related peritonitis. Routine examination of peritoneal dialysis fluid showed abdominal infection, and the results of microbial culture of the peritoneal dialysis fluid confirmed Neisseria mucosa. Imi-penem/ cilastatin 1.0 g q12h was added to peritoneal dialysis fluid for anti-infection treatment. After 24 d, the patient underwent upper extremity arteriovenous fistulation. One month later, the patient was discharged home in a clinically stable state.
Peritonitis caused by Neisseria mucosa is rare. Patients with home-based self-dialysis cannot guarantee good medical and health conditions, and require education on self-protection.
Core Tip:Neisseria mucosa is part of the normal human flora when it is parasitic in the oral and nasal mucosa, and rarely causes infection. However, it may be associated with severe disease when patients undergo invasive, instrumented procedures or have underlying conditions, as shown in the present patient who was undergoing continuous ambulatory peritoneal dialysis. In addition, the infection was also closely related to the patient’s health behavior and habits during peritoneal dialysis. Treatment should be based on antimicrobial susceptibility testing and a sufficient and full course of antimicrobial therapy should be given.
- Citation: Ren JM, Zhang XY, Liu SY. Neisseria mucosa - A rare cause of peritoneal dialysis-related peritonitis: A case report. World J Clin Cases 2023; 11(14): 3311-3316
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3311.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3311
The Gram-negative diplococci Neisseria are commensal bacteria of mucosal surfaces in humans[1]. Two major subspecies, Neisseria meningitidis and Neisseria gonorrhoeae, are pathogenic in humans, while most other subspecies such as Neisseria mucosa are recognized as non-pathogenic. Neisseria mucosa is generally not pathogenic in healthy individuals with normal immunity, but can be pathogenic in those with impaired immune function or changes in bacterial colonization sites.
It has been reported that Neisseria mucosa is associated with meningitis, endocarditis, pneumonia, bacteremia, arthritis, peritonitis, endophthalmitis and urethritis[2-7]. We herein report the first case of refractory peritoneal dialysis-related peritonitis caused by Neisseria mucosa in mainland China.
A 55-year-old Chinese female presented to the nephrology clinic with the complaint of abdominal pain for 2 d.
Symptoms started 2 d before presentation with no obvious cause of upper abdominal pain (the Visual analogue scale was 2), with nausea, vomiting of gastric contents, and diarrhea, with no chills, fever, chest tightness, shortness of breath, edema, hematemesis, or melena.
The patient was diagnosed with Stage 5 chronic kidney disease five years ago, combined with renal hypertension and renal anemia. She was treated with peritoneal dialysis for renal replacement therapy, and with compound α-keto acid tablets, amlodipine tablets, and clonidine tablets.
The patient denied a history of hepatitis, tuberculosis and other infectious diseases, diabetes, cardiovascular and cerebrovascular disease, and had no family history of malignant tumors.
On initial evaluation, vital signs revealed a temperature of 36.5 °C, pulse rate of 98 bpm, blood pressure of 116/77 mmHg and a respiration rate of 19 breaths/min. The patient was conscious and oriented without chills, diarrhea, chest tightness, chest pain or any other discomfort. No yellowing of the skin or eyes was observed. Both lungs were clear, no dry or moist crackles (rales) were heard. Furthermore, the abdomen was flat, an abdominal tube was visible on the right lower abdomen, the tunnel had a little exudation, and the tunnel score was 1 point. No tenderness, rebound pain, enlarged liver or spleen, and no tapping pain in both kidneys were observed. No edema was noted in both ankles, limb muscle strength was Grade V, and Babbitt negative.
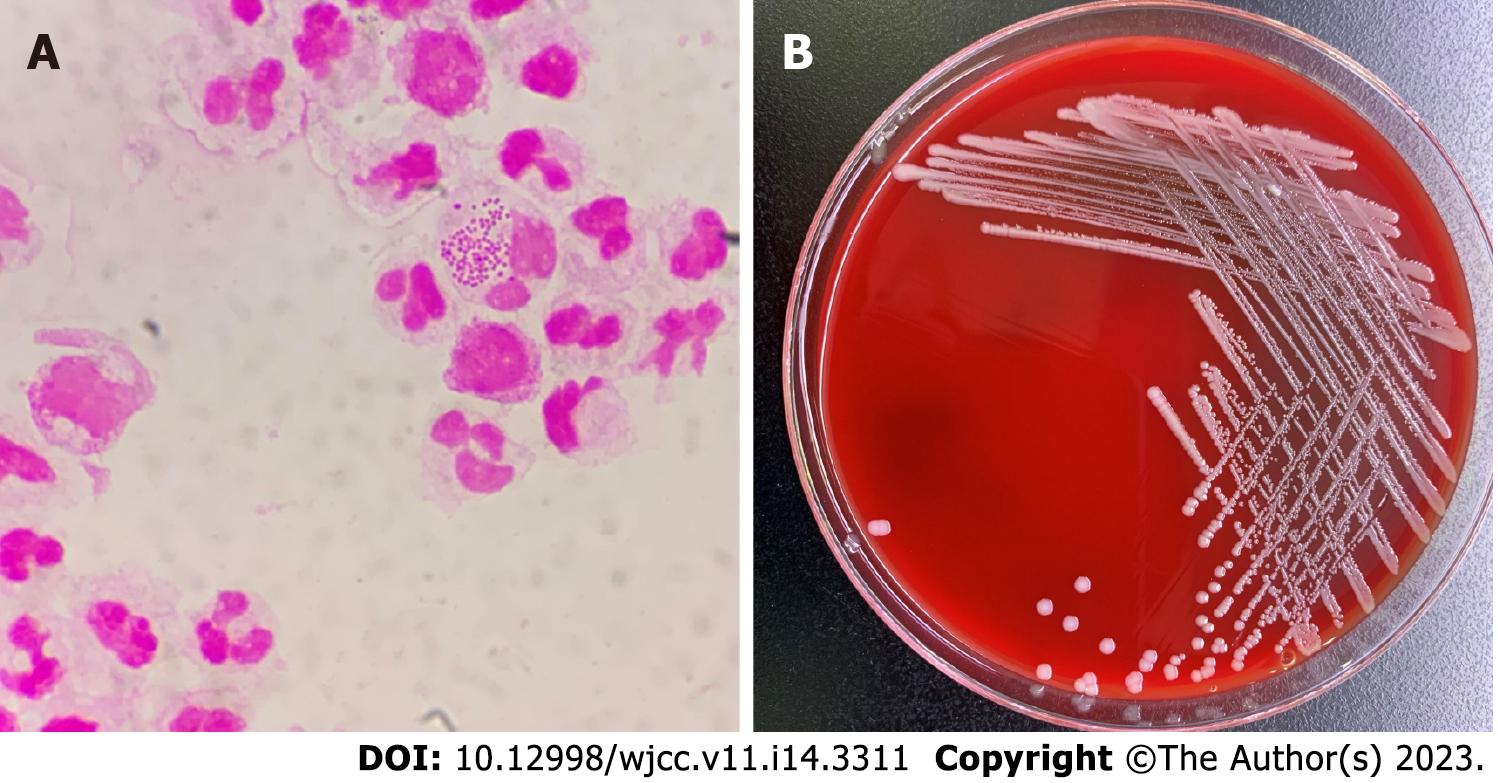
Routine blood examination showed the following results: Leukocyte count 6.0 × 109/L, neutrophil percentage 89.7%, hemoglobin 108 g/L; high sensitivity C reactive protein 244.94 mg/L. On the day of admission, routine examination of peritoneal dialysis fluid was performed after disinfection which showed that the fluid was colorless, turbid, with a white blood cell count of 6000/μL, and neutrophils of 95%. The Lifanta test was positive, and dialysate bacterial smear examination showed Gram-negative cocci identified by Gram staining (Figure 1A).
Blood electrolyte results were: Potassium 3.52 mmol/L, sodium 131.9 mmol/L, and chlorine 89.0 mmol/L. After admission, peritoneal dialysis fluid was collected for bacterial smear examination. The peritoneal dialysis fluid was cultured on a Columbia blood agar plate for 48 h, and 2-4 mm, gray-white, round, moist, and raised colonies were observed (Figure 1B). The pure culture was identified as Neisseria mucosa/mucosa by a Vitek MS mass spectrometer. In order to obtain accurate identification results, 16sRNA sequencing was performed. The results showed that the sequence was 99.85% consistent with Neisseria mucosa (GenBank No. NR_117696.1). Combined with the colony characteristics, the strain was finally identified as Neisseria mucosa.
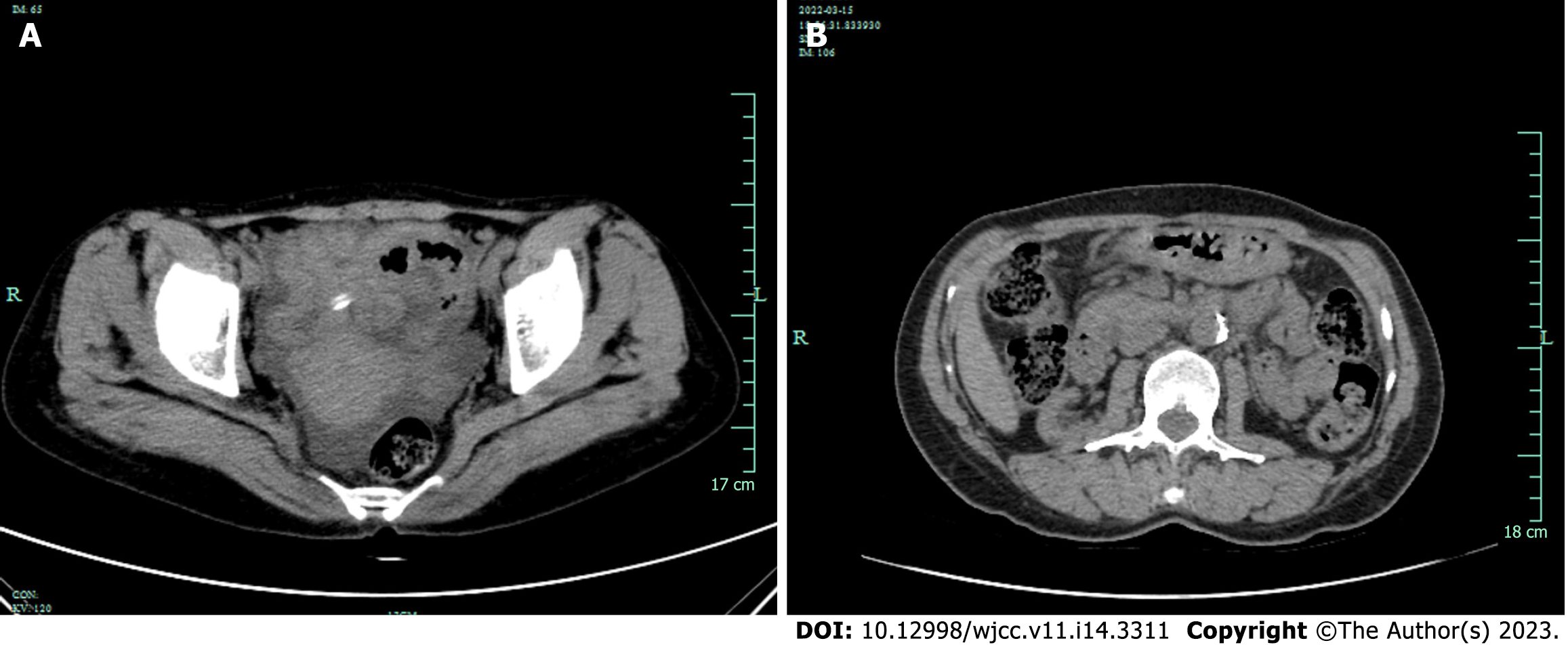
Hepatobiliary system, spleen, pancreas, kidneys and ureters were examined by brightness-mode ultrasound, and the patient was found to have a right kidney cyst and ascites. She was admitted to hospital after an initial diagnosis of peritonitis. Routine enhanced computed tomography (CT) of the abdomen and pelvis showed that the patient had multiple cysts in both kidneys and pelvic effusion. There was no evidence of intra-abdominal abscess or a focus of infection. Two weeks later, reexamination with CT showed that after treatment of peritonitis, peritoneal thickening has not been completely absorbed caused by peritonitis (Figure 2).
Combined with the patient’s medical history, the final diagnosis was peritoneal dialysis-related peritonitis caused by Neisseria mucosa.
For adequate coverage of Gram-positive and Gram-negative organisms, the empirical antibiotic therapy given was vancomycin 1000 mg qd combined with imipenem/cilastatin 1.0 g q12h added to peritoneal dialysis fluid. In addition, 2.5% dialysate 2 L + 1.5% dialysate 6 L/d continuous ambulatory peritoneal dialysis was carried out to eliminate toxins and maintain water, electrolyte, and acid-base balance. Vancomycin was discontinued after the pathogen was identified, and imipenem/cilastatin injection 1.0 g q12h for anti-infection treatment was continued. After 8 d, the patient's dialysate was clear and her condition had improved. Ceftazidime injection 1.0 g qd was added to the dialysate for follow-up anti-inflammatory treatment.
On the 24th day of hospitalization, arteriovenous fistulation of the upper extremity was performed. The purpose of surgical treatment is to change his treatment plan from peritoneal dialysis to hemodialysis three months after the operation. The patient was discharged in a stable condition after one month. Two weeks after discharge, the patient was again hospitalized due to peritoneal dialysis-related peritonitis. The same organism-Neisseria mucosa was cultured from the patient's peritoneal dialysis fluid. After a detailed medical history enquiry, it was found that the patient frequently used her mouth to open the cap of the dialysate bottle during peritoneal dialysis at home. During her second hospitalization, the patient was treated with imipenem/cilastatin injection 1.0 g q12h added to peritoneal dialysis fluid for 20 d and was cured. The patient is currently undergoing close follow-up observation.
Neisseria species other than Neisseria meningitidis and Neisseria gonorrhoeae, such as Neisseria mucosa, Neisseria subflava, Neisseria lactamica, Neisseria cinerea, and Neisseria mucosa, are unusual pathogens in humans[8]. Neisseria mucosa, originally described as Diplococcus mucosus by von Lingelsheim in 1906, frequently colonizes the nasopharynx and dolphin respiratory tract[9]. It was gram-negative diplo
The patient was diagnosed with Stage 5 chronic kidney disease, combined with renal hypertension, renal anemia, and maintenance peritoneal dialysis for more than 5 years. Therefore, the patient's immune function and nutritional status were poor, and the peritoneal self-defense function was impaired. Combined with the significant increase in systemic infection indicators, the leukocytes in peritoneal dialysis fluid were chemotactic and were mainly polymorphonuclear neutrophils. Bacterial smears and culture results confirmed the diagnosis of peritoneal dialysis-associated peritonitis caused by Neisseria mucosa.
The patient was treated with antibiotics added to peritoneal dialysis fluid for anti-inflammatory treatment. According to the Clinical and Laboratory Standards Institute[3], there is no accepted standard for assessing the antimicrobial sensitivity of Neisseria mucosa. We provide in vitro drug sensitivity test results for reference: Penicillin G (0.125 μg/mL), ceftriaxone (0.25 μg/mL), meropenem (0.125 μg/mL), combined with the International Society of Peritoneal Dialysis (ISPD) guidelines for the treatment of abdominal infection[15], the first treatment with imipenem/cilastatin resulted in significant improvement, and 8 d after treatment with ceftazidime, the clinical symptoms disappeared.
However, the patient was readmitted to hospital due to peritonitis caused by Neisseria mucosa two weeks later, according to ISPD peritonitis guideline recommendations in 2022[16]. The patient's second infection was defined as a relapse. The reason for the second infection may be that the first treatment did not involve removal of the peritoneal dialysis tube, resulting in a small amount of bacterial colonization. In addition, after a detailed medical history enquiry, it was found that the patient frequently used her mouth to open the cap of the dialysate bottle during peritoneal dialysis at home. Therefore, it is speculated that the patient's Neisseria mucosa was derived from the peritoneal dialysis fluid contaminated by her own saliva and caused repeat peritonitis.
In summary, we report a case of peritoneal dialysis-related peritonitis caused by repeated infection with Neisseria mucosa. There are few reports on infections caused by Neisseria mucosa. The pathogenic mechanism, infection route and treatment plan of Neisseria mucosa require further study. Peritoneal dialysis patients, due to long-term impaired renal function and low immunity, should pay attention to the prevention of peritonitis. In particular, in home-based self-dialysis patients, medical and health conditions are poor; thus, education on relevant protective measures is needed.
Peritoneal dialysis patients, due to long-term impaired renal function and low immunity, should pay attention to the prevention of peritonitis. Neisseria mucosa, as part of the normal flora of the human oral cavity, rarely causes infection in the body, especially the abdominal cavity. Peritonitis caused by Neisseria mucosa is very rare. Neisseria mucosa was detected during two hospitalizations for peritonitis in this case, which may have been related to the patient's habit of opening the peritoneal dialysis fluid cap using her mouth. Unfortunately, we were unable to confirm whether Neisseria mucosa from the patient's mouth was the same strain as the two isolated strains. However, this case demonstrates that home-based self-dialysis patients, because they cannot guarantee good medical and health conditions, need to be repeatedly educated and protected.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA, Brazil; Suwanto D, Indonesia S-Editor: Hu YR L-Editor: A P-Editor: Chen YX
| 1. | Zapun A, Morlot C, Taha MK. Resistance to β-Lactams in Neisseria ssp Due to Chromosomally Encoded Penicillin-Binding Proteins. Antibiotics (Basel). 2016;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Altdorfer A, Pirotte BF, Gaspard L, Gregoire E, Firre E, Moerman F, Moonen M, Sanoussi A, Van Esbroeck M, Mori M. Infective endocarditis caused by Neisseria mucosa on a prosthetic pulmonary valve with false positive serology for Coxiella burnetii - The first described case. IDCases. 2021;24:e01146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Khan KN, Saxena R, Choti M, Ariyamuthu VK. Neisseria mucosa Peritonitis in the Setting of a Migrated Intrauterine Device. Adv Perit Dial. 2018;34:47-49. [PubMed] [Cited in This Article: ] |
| 4. | Osses DF, Dijkmans AC, van Meurs AH, Froeling FM. Neisseria Mucosa: A New Urinary Tract Pathogen? Curr Urol. 2017;10:108-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Mechergui A, Achour W, Baaboura R, Ouertani H, Lakhal A, Torjemane L, Othman TB, Hassen AB. Case report of bacteremia due to Neisseria mucosa. APMIS. 2014;122:359-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Durán E, Salvo S, Gil J, Sanjoaquín I. [Pneumonia and bacteremia due to Neisseria mucosa in a human immunodeficient virus seropositive patient parenteral drug abuser]. Enferm Infecc Microbiol Clin. 2011;29:236-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Gini GA. Ocular infection in a newborn caused by Neisseria mucosa. J Clin Microbiol. 1987;25:1574-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | John EB, Raphael D, Martin JB. Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 9th ed. Elsevier, 2019: 3049-3135. [Cited in This Article: ] |
| 9. | Jin Y, Xu H, Yao Q, Gu B, Wang Z, Wang T, Yu X, Lu Y, Zheng B, Zhang Y. Confirmation of the Need for Reclassification of Neisseria mucosa and Neisseria sicca Using Average Nucleotide Identity Blast and Phylogenetic Analysis of Whole-Genome Sequencing: Hinted by Clinical Misclassification of a Neisseria mucosa Strain. Front Microbiol. 2021;12:780183. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 10. | Arora S, Chitkara NL. Non-pathogenic neisserian Neisseria catarrhalis--as cause of meningitis. J Assoc Physicians India. 1973;21:255-257. [PubMed] [Cited in This Article: ] |
| 11. | Macia M, Vega N, Elcuaz R, Aterido T, Palop L. Neisseria mucosa peritonitis in CAPD: another case of the "nonpathogenic" Neisseriae infection. Perit Dial Int. 1993;13:72-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Lee WC, Yang WC, Chen TW, Huang CH, Lin CC. Unusual presentation of Neisseria mucosa peritonitis with persistent ultrafiltration failure and clear effluent. Perit Dial Int. 2003;23:198-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Shetty AK, Nagaraj SK, Lorentz WB, Bitzan M. Peritonitis due to Neisseria mucosa in an adolescent receiving peritoneal dialysis. Infection. 2005;33:390-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Awdisho A, Bermudez M. A Case Report of Neisseria Mucosa Peritonitis in a Chronic Ambulatory Peritoneal Dialysis Patient. Infect Dis Rep. 2016;8:6950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J Clin Microbiol. 2021;59:e0021321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 228] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 16. | Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, Kanjanabuch T, Kim YL, Madero M, Malyszko J, Mehrotra R, Okpechi IG, Perl J, Piraino B, Runnegar N, Teitelbaum I, Wong JK, Yu X, Johnson DW. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 148] [Article Influence: 74.0] [Reference Citation Analysis (0)] |