Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1056
Peer-review started: June 21, 2021
First decision: July 26, 2021
Revised: August 20, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 21, 2022
Mutations in the beta1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2) gene can lead to impaired glycosylation of α-dystroglycan, which, in turn, causes congenital muscular dystrophy (CMD). The clinical phenotypes of CMD are broad, and there are only a few reports of CMD worldwide.
This report describes the cases of two children with CMD caused by B3GALNT2 gene mutation. The main manifestations of the two cases were abnormal walking posture, language development delay, and abnormal development of the white matter. Case 2 also had unreported symptoms of meningocele and giant arachnoid cyst. Both cases had compound heterozygous mutations of the B3GALNT2 gene, each containing a truncated mutation and a missense mutation, and three of the four loci had not been reported. Nineteen patients with CMD caused by B3GALNT2 gene mutation were found in the literature. Summary and analysis of the characteristics of CMD caused by B3GALNT2 gene mutation showed that 100% of the cases had nervous system involvement. Head magnetic resonance imaging often showed abnormal manifestations, and more than half of the children had eye and muscle involvement; some of the gene-related symptoms were self-healing.
B3GALNT2 gene can be used as one of the candidate genes for screening CMD, cognitive development retardation, epilepsy, and multiple brain developmental malformations in infants.
Core Tip: Mutations in the beta1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2) gene can lead to impaired glycosylation of α-dystroglycan, which, in turn, causes congenital muscular dystrophy (CMD). The clinical phenotypes of CMD are broad, and there are only a few reports of CMD worldwide. This paper introduces two cases of congenital dystrophy caused by mutation of the B3GALNT2 gene. Briefly, 19 children with B3GALNT2 gene mutation published in the world were reviewed. Clinical characteristics and mutation genotypes of 21 children were analyzed, and the pathogenesis is discussed.
- Citation: Wu WJ, Sun SZ, Li BG. Congenital muscular dystrophy caused by beta1,3-N-acetylgalactosaminyltransferase 2 gene mutation: Two case reports. World J Clin Cases 2022; 10(3): 1056-1066
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1056.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1056
Congenital muscular dystrophy (CMD) refers to the primary and progressive myopathy that occurs at birth or within a few months after birth, usually causing considerable burden to the child and their families and society. The main clinical manifestations of CMD include premature myasthenia, hypodystonia, motor development retardation, and joint contracture with or without central nervous system involvement. The creatinase level tends to increase to varying degrees, myogenic damage can be seen on electromyography, and muscular dystrophy is the most common muscle pathology. Ge et al[1] reported that the prevalence rate of CMD in China was 0.017-0.083/100000. CMD is a group of hereditary diseases with strong heterogeneity caused by anti-dystroglycan (DG) deficiency. DG is a protein that is widely expressed in a variety of tissues, including the brain, Schwann cells of peripheral nerves, neuromuscular junction, muscle, liver, lung, and skin[2]. DG is cleaved into α-DG and β-DG after translation. The core structure of α-DG is dumbbell-shaped, with a spherical C-terminal and an N-terminal at the two ends, respectively, while the middle is connected by a mucoprotein-like structure. α-DG undergoes O-linked glycosylation, which rarely occurs in vivo[3], and then forms an anti-dystrophin-glycoprotein complex with β-DG. Then, it binds with ligands containing a laminin globular domain in the extracellular matrix, such as laminin, perlecan, agglutinin, axon protein, and pikacurin, to maintain muscle cell integrity[4], thus playing an important role in brain development[5]. α-DG has many glycosylation sites, short glycoprotein fragments, complex functions, and diverse pathogenic genes[6]. Once the glycosylation of α-DG is impaired, it can trigger many pathophysiological processes, such as cell migration disorder[7] or α-dystroglycanopathy (α-DGP)[8]. Recent studies[9] have shown that the gene located in 1q42.3 containing 64292 base pairs is one of its pathogenic genes, which encode beta1,3-N-acetylgalactosaminyltransferase2 (B3GALNT2). The deficiency of this enzyme can lead to the damage of α-DG glycosylation, consequently impairing the function of α-DG and affecting cell proliferation, migration, differentiation, and maintenance of tissue integrity, resulting in α-DGP[10]. Its pathogenic type was autosomal recessive inheritance.
At present, there are few reports about the B3GALNT2 gene causing CMD, and the relationship between genotype and phenotype is still not fully understood. In this paper, the pathogenic characteristics of this gene in two cases of α-DGP caused by B3GALNT2 gene mutation found in our hospital are summarized and discussed in view of previous reports.
Case 1: A 49-mo-old Chinese Han boy presented with abnormal walking posture in January 2019.
Case 2: A 45-mo-old Chinese Han boy presented with delayed growth and development from childhood and an abnormal scalp in June 2019.
Case 1: The child showed signs of developmental delay since childhood. He could raise his head at 5 mo, sit at 8 mo, walk alone at 1 year and 8 mo, and say the Chinese words for “mother” and “father” at 28 mo.
Case 2: The child could raise his head at 6 mo, sit alone at 12 mo, and walk at 24 mo. At the time of this visit, he still walked with a spastic gait. At present, he could pronounce a small number of disyllabic words such as the Chinese words for “father” and “mother”.
Case 1: The patient had no special past history.
Case 2: Meningocele was seen in the parietal and occipital region after birth, which then gradually healed.
Case 1: The child was the fifth fetus and the second delivered by cesarean section at 38 wk of pregnancy. There was no significant family history.
Case 2: The child was the second fetus and delivered by full-term natural delivery. There was no significant family history.
Case 1: Physical examination revealed that the child could understand and respond to instructions, walked with “duck step”, and had no skin abnormalities or abnormal palpebral fissures. The bilateral pupils were round, equal in size, and sensitive to light reflex. The patient had no stiff neck, and no obvious heart, lung, and abdomen abnormalities. He had normal muscle strength of the extremities, bilateral biceps reflex, knee-tendon reflex, and active Achilles tendon reflex. He had low muscle tone and positive bilateral Babinski signs.
Case 2: Physical examination revealed that the child could understand and respond to instructions well, and walked with a spastic gait. An abnormal area of approximately 3 cm × 2 cm was visible in the parietal occipital area of the scalp; it was blue and purple in color, lacked hair growth, and showed no rupture, no tenderness, and no fluctuation. The bilateral eye fissure had no deformity, and bilateral pupils were round, equal in size, and sensitive to light reflex. There were no limitations of eye movement; no stiff neck; and no obvious abnormalities in the heart, lung, and abdomen. The muscle strength of the extremities was normal, and muscle tension was high. Bilateral biceps reflex, knee-tendon reflex, and Achilles tendon reflex were active, and bilateral Babinski signs were negative.
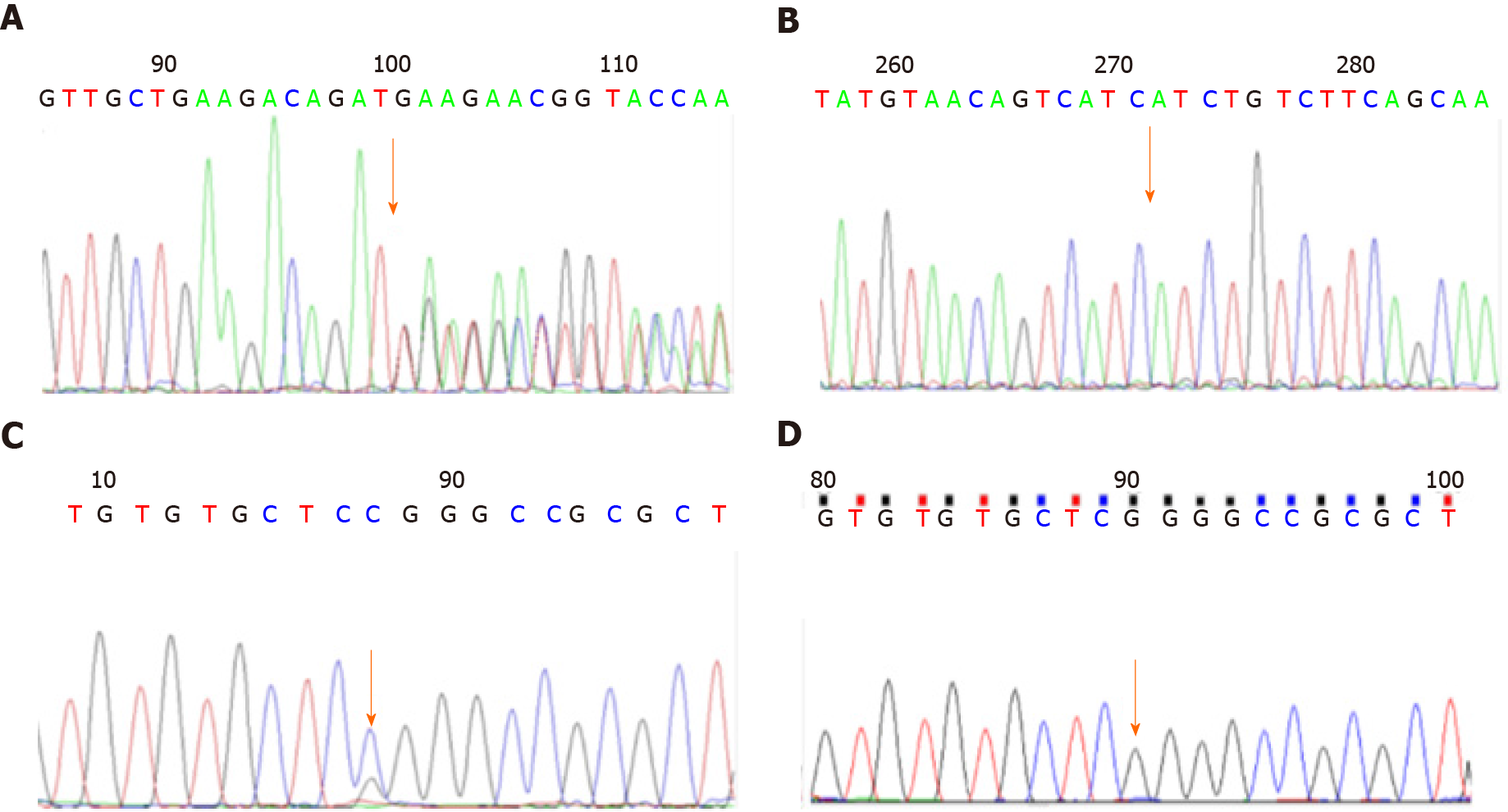
Case 1: The leukoencephalopathy gene package (McKinnon) was used for sequencing, and the results demonstrated that the B3GALNT2 gene had a compound heterozygous mutation (Figure 1), and the allele mutations included c.1068dupT (p.D357_D358 delinsX) from the father (Figures 1A and 1B) and c.40G>C (p.G14R) from the mother (Figures 1C and 1D). The former was a nonsense mutation, which led to protein truncation, and the latter was a missense mutation.
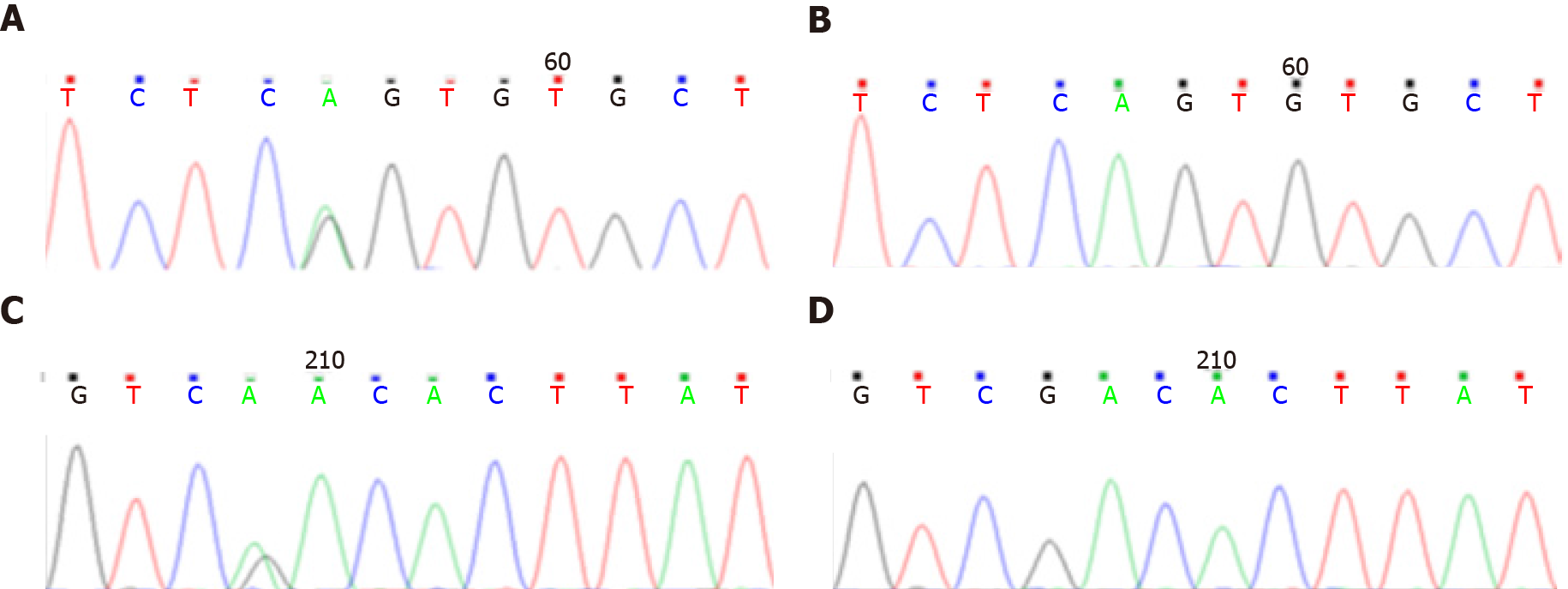
Case 2: Genetic examination showed that the B3GALNT2 carried a compound heterozygous mutation (Figure 2). The mutation sites on the allele included c.261-2A>G (splicing) from the mother (Figures 2A and 2B) and c.979G>A (p.D327N) from the father (Figures 2C and 2D). The former was a missense mutation, and the latter was a nonsense mutation, which led to protein truncation.
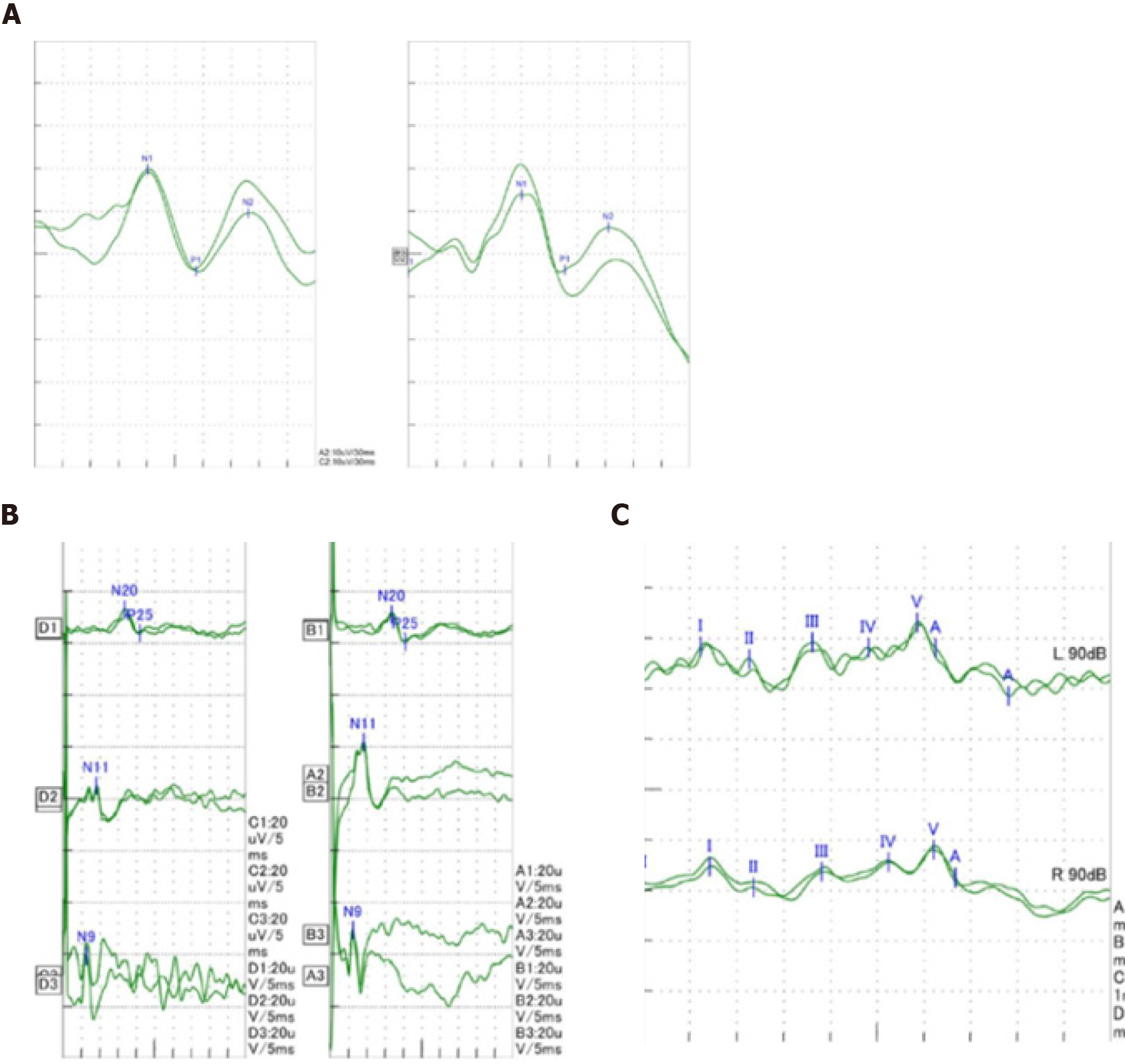
Case 1: At the age of 2 years and 4 mo, electromyography of the extremities was generally normal. Visual evoked potentials (Figure 3A) showed that both eyes were stimulated, each wave was well-differentiated, and P1 latency was significantly prolonged. Somatosensory evoked potentials (Figure 3B) showed that both upper limbs were stimulated, the cortical and surrounding waves were well-differentiated, and the latencies were roughly normal. Auditory evoked potentials (Figure 3C) suggested that bilateral ears were stimulated, the waveform of the left side was relatively poor, the latencies of the right III, IV, and V waves were prolonged. Head magnetic resonance imaging (MRI) showed cerebellar cortical cysts (Figure 4A), multiple flaky and linear equal/slightly long T1, long T2, and high fluid-attenuated inversion recovery signals around the bilateral ventricles of the brain (Figures 4B and 4C).
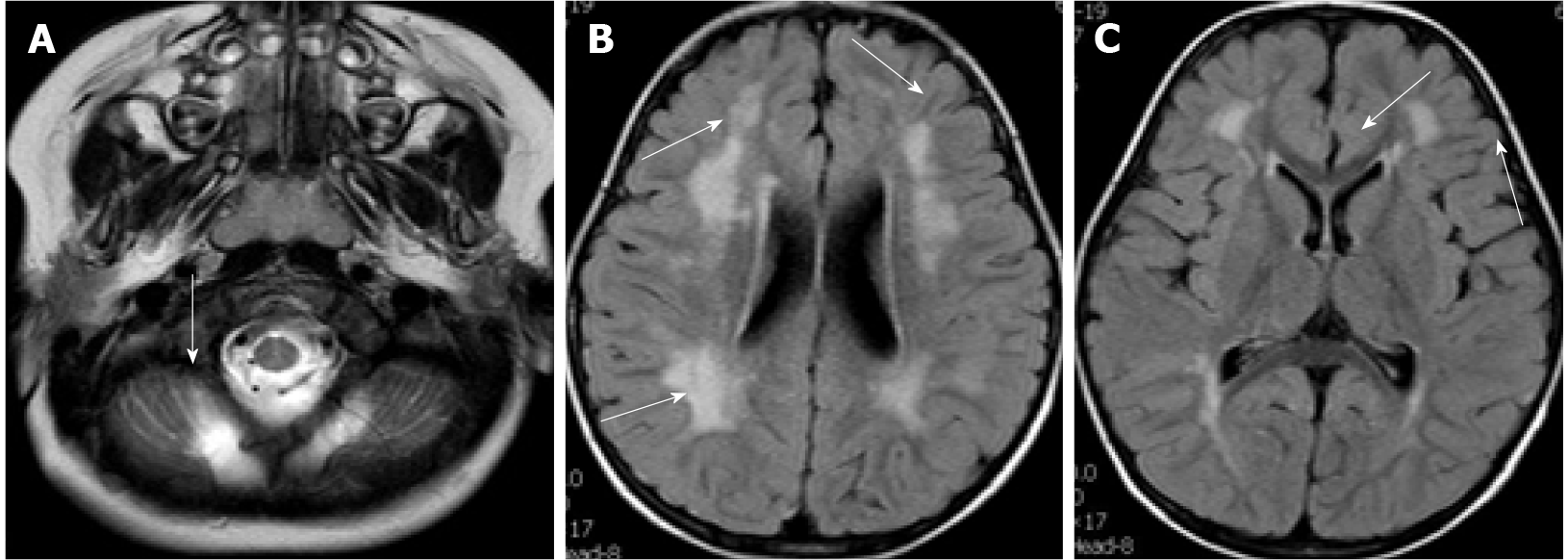
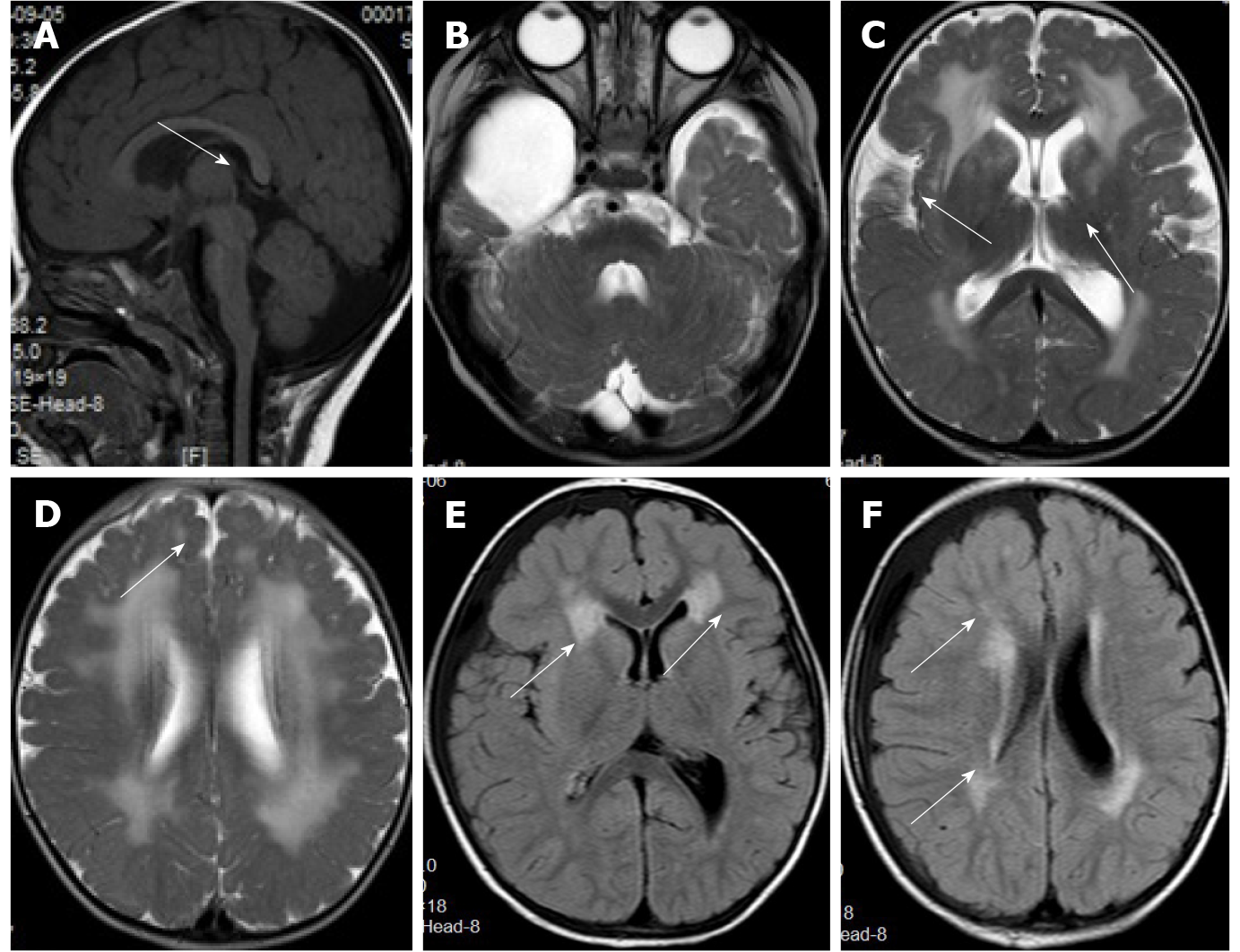
Case 2: The first head MRI (Figure 5) at 10 mo of age showed an abnormal signal of the flat pons (Figure 5A), an arachnoid cyst in the right temporal pole (Figure 5B), and periventricular white matter neuronal migration disorder in bilateral frontal and parietal lobes-polymicrogyria malformation (Figures 5C and 5D). The second head MRI at 2 years and 11 mo revealed an abnormal signal of periventricular white matter (Figures 5E and 5F), which was absorbed and considerably improved compared to that seen in the previous MRI; neuronal migration disorder in bilateral frontotemporal parietal lobes-polymicrogyria malformation, an arachnoid cyst in the right temporal pole, and a flat pons.
The final diagnosis for both cases was B3GALNT2 gene mutation-related α-DGP. Case 2 was also diagnosed with a giant arachnoid cyst.
Both cases received coenzyme Q10 and levocarnitine. The giant arachnoid cyst in case 2 was resected.
The patient could pronounce 2- to 3-syllable words, and his walking posture was improved over the course of the follow-up.
At the latest follow-up visit, the child’s walking posture was improved compared to before, although language development did not significantly improve. He recovered well after resection of the giant arachnoid cyst, and the meningocele gradually self-healed.
A total of 21 articles were found using the search key word ”B3GALNT2” on the PubMed website, of which five articles were related to human CMD. The first article was published in 2013. The five articles included four pedigrees and seven sporadic patients. A total of 21 patients were reported, including the two patients in the present case report. Tables 1 and 2 present a summary of all these cases: (1) The nervous system was involved in all cases, and cognitive and motor developmental disorders were the most commonly encountered clinical symptoms (100%), followed by seizures (52.6%) and low muscle tension (64.3%); (2) Abnormal head MRI may be manifested as periventricular white matter lesion (81.3%), neuronal migration disorder (68.8%), brainstem and/or cerebellar dysplasia (56.3%), cerebellar cortical cyst (50%), and severe hydrocephalus (31.25%); (3) Muscle involvement was seen in 63.2% of patients, and the increased creatinase level ranged from 300 to 1740 U/L, which was even higher in patients with a more severe condition; and (4) Eye involvement occurred in 28.6% of the cases. The common symptoms were optic nerve atrophy and eye fissure deformities. In severe cases, glaucoma, cataract, and even blindness could be observed.
| Source in the order of report time | Serial number | Gender | Nervous system (21, 100%) | Eyes (6, 28.6%) | Muscles (12, 63.2%) | ||||
| Development of movement and cognition is lagging behind (21, 100%) | Epilepsy (9, 52.6%) | Low muscle tension (11, 64.3%) | Optic nerve atrophy | Palpebral fissure deformity | Others | Increased creatinase (maximum creatinase value or range) U/L | |||
| 2013 Stevens et al[9] | Sporadic 1 | Male | + | - | - | + | - | - | + (1132) |
| Sporadic 2 | Male | + | - | - | - | - | - | + (894) | |
| Sporadic 3 | Female | + | + | + | + | - | Blo | + (21000) | |
| Pedigree; 5; 1 | Male | + | + | + | - | + | Rcg, lc | + (6964) | |
| Female | + | + | + | NA | NA | B | (NA) | ||
| Sporadic 6 | Female | + | - | - | - | - | NA | + (1740) | |
| Sporadic 7 | Male | + | - | + | - | + | - | + (1086) | |
| 2014 Hedberg et al[11] | Sporadic 8 | Female | + | - | + | - | - | - | + (647) |
| 2017 Ho et al[12] | Pedigree 2 9 | Male | - | - | + | - | - | - | + (300-900) |
| 10 | Male | - | - | + | - | - | - | ||
| 11 | Unknown | - | - | + | - | - | - | ||
| 2017 Maroofian et al[13] | Pedigree 3 | Male | + | - | - | - | - | - | - |
| 12; 13 | Male | + | - | - | - | - | - | NA | |
| Pedigree | Male | + | + | NA | - | - | - | - | |
| 4 | Male | + | + | NA | - | - | - | - | |
| Female | + | + | NA | - | - | - | - | ||
| 14 | Female | + | + | NA | - | - | - | - | |
| 15; 16; 17; 18 | Female | + | + | NA | - | - | - | - | |
| 2018 AI et al[14] | Sporadic 19 | Female | + | + | + | NA | + | B | + (2565) |
| This report | Sporadic 20 | Male | + | - | + | - | - | - | - |
| Sporadic 21 | Male | + | - | + | - | - | - | + (952) | |
| Source in the order of report time | Serial number | Head MRI | Mutant genotype | |||||
| Hydrocephalus (5, 31.3%) | White matter lesions (13, 81.3%) | Cerebellar cortical cyst (8, 50%) | Neuronal migration disorder (polymicrogyria and cobblestone-like no gyrus) (11, 68.8%) | Brain stem and/or cerebellum dysplasia (9, 56.3%) | ||||
| 2013 Stevens et al[9] | Sporadic 1 | - | + | + | + | - | c.740G>A (p.G247E); c.875G>C (p.R292P) | |
| Sporadic 2 | - | + | + | + | + | c.51_73dup; p.S25Cfs*38 (homozygous) | ||
| Sporadic 3 | + | - | - | + | + | c.726_727del (p.V243Efs*2); c.822_823dup (p.I276Lfs*26) | ||
| 1 | 4 | + | - | - | + | - | c.308_309del (p.V103Gfs*10); c.755T>G (p.V252E) | |
| Pedigree 1 | 5 | + | + | - | + | - | NA | |
| Sporadic 6 | - | + | - | - | + | c.802G>A (homozygous) (p.V268M) | ||
| Sporadic 7 | + | - | - | + | - | c.1423C>T (homozygous) (p.Gln475*) | ||
| 2014 Hedberg et al[11] | Sporadic 8 | - | + | + | - | + | c.192dupT (p.Efs*); c.979G>A (p.D327N) | |
| 2017 Ho et al[12] | Pedigree 2 | 9 | - | + | + | + | + | NA |
| 10 | - | + | + | + | + | |||
| 11 | - | + | + | + | + | |||
| 2017 Maroofian et al[13] | Pedigree 3 | 12 | - | + | - | - | - | c.822_823dup (p.I276Lfs*263); c.988C>T (p.R330C) |
| 13 | NA | NA | NA | NA | NA | |||
| Pedigree 4 | 14 | - | + | - | - | - | c.979G>A (homozygous) (p.D327N) | |
| 15 | NA | NA | NA | NA | NA | |||
| 16 | NA | NA | NA | NA | NA | |||
| 17 | NA | NA | NA | NA | NA | |||
| 18 | NA | NA | NA | NA | NA | |||
| 2018 AI et al[14] | Sporadic 19 | + | + | - | + | + | c.448C>T (homozygous) (p.R150*) | |
| This report | Sporadic 20 | - | + | + | - | - | c.1068dup T (p.D357_ D358delin sX); c.40G>C (p.G14R) | |
| Sporadic 21 | - | + | + | + | + | c.261-2A>G (splicing); c.979G>A (p.D327N) | ||
This study reports two cases of CMD caused by B3GALNT2 gene mutation. Both cases had an abnormal gait and significant delays in language development. Head MRI was performed for both children and revealed the presence of white matter lesions in the brain. The present report suggested that the cognitive language retardation and white matter lesions in CMD were caused by B3GALNT2 gene mutation. New symptoms of meningocele and giant arachnoid cyst were also encountered in one of our cases. To our knowledge, this is the first study to use electrophysiological examination of the audiovisual conduction pathway in children with this type of gene mutation. The findings suggest that even if audiovisual function was normal, the evoked audiovisual potential should be examined to investigate for potential abnormalities. The white matter lesions in case 2 were gradually absorbed and improved with age. He recovered well after surgical resection of the giant arachnoid cyst, and the meningocele gradually self-healed. These results suggested that some of the symptoms associated with the gene mutation might have a tendency to self-heal, which was consistent with the report of Hedberg et al[11]. Further studies are required to determine whether this is related to the peak age of gene expression. Both of the present cases had compound heterozygous mutations, the gene mutation sites were from their parents, and the mutations were compatible with autosomal recessive inheritance.
Case 1 had two compound heterozygous mutations in the B3GALNT2 gene; one of them was c.1068dupT (p.D357_D358delinsX), which was a nonsense (zero-effect) mutation that may lead to loss of gene function. The frequency in the normal population database was 0.00010, indicating that this was a low-frequency variation (PM2). The bioinformatics protein function prediction software packages SIFT, PolyPhen_2, and REVEL all predicted it as unknown. According to the American College of Medical Genetics and Genomics (ACMG) guidelines, the variation was preliminarily determined as a suspected pathogenic variation. The other was c.40G>C (p.G14R), which was a missense mutation. It was (-) in the normal population database, which indicated a low-frequency variation (PM2). It existed in trans with another pathogenic variation (PM3). SIFT, PolyPhen_2, and REVEL predicted it as harmful, harmful, and benign, respectively. According to the ACMG guidelines, the variation was preliminarily determined as being of unknown clinical significance. In case 2, there were two compound heterozygous mutations in the B3GALNT2 gene: One was c.261-2A>G, which was located in the classical splicing site (± 1, 2). It was likely to affect the normal splicing of mRNA and lead to protein truncation. The frequency in the normal population database was 0.00004, which showed that this was a low-frequency variation (PM2). According to the ACMG guidelines, this variation was preliminarily determined to be a suspected pathogenic variation (PS1). The other site was c.979G>A (p.D327N), which was a known PS1 in the Human Gene Mutation Database, and the frequency of variation in the normal population was 0.00003 (PM2). In the transposition, the suspected pathogenicity variation c.261-2A>G (PM3) was detected. SIFT, PolyPhen2, and Mutation Taster all predicted that the mutation was harmful (PP3). According to the ACMG guidelines, the variation was preliminarily determined to be a suspected PS1. According to the clinical symptoms and gene results, both children were diagnosed as having CMD caused by the B3GALNT2 gene mutation. Each child had one missense mutation, and each had a mutation leading to protein truncation. Case 1 had a frameshift mutation leading to protein truncation, whereas case 2 had a splicing mutation leading to protein truncation, suggesting that truncation mutation may be related to a severe clinical phenotype.
In this study, data from two hospitalized patients and data of 19 patients obtained from a search of the previous literature were collected, yielding data from a total of 21 patients with α-DGP caused by B3GALNT2 gene mutation. Clinical manifestations included common symptoms such as nervous system, muscular dystrophy, eye development malformation, and rare symptoms such as sensorineural deafness. Moreover, 100% of the 21 patients had nervous system involvement, and delayed cognitive and motor development. In severe cases, patients had no cognitive and motor development, and most cognitive disorders included language retardation. Patients with a mild lesion on MRI only had periventricular white matter lesions, while patients with severe lesions seen on MRI had brainstem and/or cerebellar dysplasia and neuronal migration disorders. Among the 21 patients, 63.2% had elevated creatinase that was mostly between 300 and 1740 U/L, and 26.6% of the patients had eye involvement. The common symptoms were optic nerve atrophy and eye fissure deformities (excessive small or large). Patients with extensive nervous system and muscle involvement had severe ocular symptoms such as glaucoma, cataracts, and even blindness.
There were 12 pairs of alleles in 21 patients involving 15 Loci. Among them, c.979G>A (p.D327N) appeared three times, and c.822_823dup (pI276Lfs*26) appeared twice, suggesting that the above two sites may be focal mutations. It was further speculated that the severity of the B3GANLT2 gene mutation was still related to its mutation type and site. All four patients with biallelic truncated mutations in sporadic cases 2, 3, 7, and 19 had neuronal migration disorders (polymicrogyria or cobblestone-like surface with no gyri). Pedigree 1 and sporadic cases 8 and 21 had severe truncated mutations of a single allele (the sites were all before the 103rd amino acid), and they also had neuronal migration disorders or developmental brain malformations. Pedigree 3 and sporadic case 20 had mild truncated mutations of a single allele (the sites were all after the 276th amino acid), and they had only white matter involvement. Other forms of mutation had relatively mild symptoms. The above results suggested that patients with a truncated mutation of the gene located more at the front of the mutation site had more severe symptoms. The missense mutation was often associated with relatively mild symptoms. Sporadic type 1 is special, in that it is a compound heterozygous missense mutation that is associated with severe symptoms such as polymicrogyria malformation and eye and muscle involvement. Further studies are required to study the mechanism underlying sporadic type 1 mutation.
This article reports two cases of CMD caused by B3GALNT2 gene mutation. Each case carried a truncated mutation and a missense mutation, while three of the four variants have not been previously reported; thus, our findings in this case report expand the gene database. Case 2 had two new phenotypes: Giant arachnoid cyst, which recovered well after surgical resection, and meningocele after birth, which gradually self-healed. These data broadened the phenotypic spectrum of the gene. Clinical characteristics of the gene mutation are summarized and analyzed through a literature search, providing clues for clinicians to diagnose and treat this gene mutation. Due to the limitation of a small number of patients, absence of functional verification, and lack of evidence at the cellular level, the clinical characteristics and pathogenic mechanisms of this mutation were not completely clarified. Further studies at a clinical level are required to support our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beran RG, Nag DS S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Ge L, Zhang C, Wang Z, Chan SHS, Zhu W, Han C, Zhang X, Zheng H, Wu L, Jin B, Shan J, Mao B, Zhong J, Peng X, Cheng Y, Hu J, Sun Y, Lu J, Hua Y, Zhu S, Wei C, Wang S, Jiao H, Yang H, Fu X, Fan Y, Chang X, Bao X, Zhang Y, Wang J, Wu Y, Jiang Y, Yuan Y, Rutkowski A, Bönnemann CG, Wei W, Wu X, Xiong H. Congenital muscular dystrophies in China. Clin Genet. 2019;96:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Nickolls AR, Bönnemann CG. The roles of dystroglycan in the nervous system: insights from animal models of muscular dystrophy. Dis Model Mech. 2018;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Sheikh MO, Halmo SM, Wells L. Recent advancements in understanding mammalian O-mannosylation. Glycobiology. 2017;27:806-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Cataldi MP, Lu P, Blaeser A, Lu QL. Ribitol restores functionally glycosylated α-dystroglycan and improves muscle function in dystrophic FKRP-mutant mice. Nat Commun. 2018;9:3448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Früh S, Romanos J, Panzanelli P, Bürgisser D, Tyagarajan SK, Campbell KP, Santello M, Fritschy JM. Neuronal Dystroglycan Is Necessary for Formation and Maintenance of Functional CCK-Positive Basket Cell Terminals on Pyramidal Cells. J Neurosci. 2016;36:10296-10313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Fu X-N, Xiao K-H, Xiong H. Recent Advances in α-Dystroglycanopathy. Prog Physiol Sci. 2019;050:10-18. [Cited in This Article: ] |
| 7. | Palmieri V, Bozzi M, Signorino G, Papi M, De Spirito M, Brancaccio A, Maulucci G, Sciandra F. α-Dystroglycan hypoglycosylation affects cell migration by influencing β-dystroglycan membrane clustering and filopodia length: A multiscale confocal microscopy analysis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2182-2191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Taniguchi-Ikeda M, Morioka I, Iijima K, Toda T. Mechanistic aspects of the formation of α-dystroglycan and therapeutic research for the treatment of α-dystroglycanopathy: A review. Mol Aspects Med. 2016;51:115-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, Tambunan DE, Yau S, Brodd L, Sewry CA, Feng L, Haliloglu G, Orhan D, Dobyns WB, Enns GM, Manning M, Krause A, Salih MA, Walsh CA, Hurles M, Campbell KP, Manzini MC; UK10K Consortium, Stemple D, Lin YY, Muntoni F. Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of α-dystroglycan. Am J Hum Genet. 2013;92:354-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Xiong H, Wang S, Kobayashi K, Jiang Y, Wang J, Chang X, Yuan Y, Liu J, Toda T, Fukuyama Y, Wu X. Fukutin gene retrotransposal insertion in a non-Japanese Fukuyama congenital muscular dystrophy (FCMD) patient. Am J Med Genet A. 2009;149A:2403-2408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Hedberg C, Oldfors A, Darin N. B3GALNT2 is a gene associated with congenital muscular dystrophy with brain malformations. Eur J Hum Genet. 2014;22:707-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Ho ML, Glenn OA, Sherr EH, Strober JB. Serial prenatal and postnatal MRI of dystroglycanopathy in a patient with familial B3GALNT2 mutation. Pediatr Radiol. 2017;47:884-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Maroofian R, Riemersma M, Jae LT, Zhianabed N, Willemsen MH, Wissink-Lindhout WM, Willemsen MA, de Brouwer APM, Mehrjardi MYV, Ashrafi MR, Kusters B, Kleefstra T, Jamshidi Y, Nasseri M, Pfundt R, Brummelkamp TR, Abbaszadegan MR, Lefeber DJ, van Bokhoven H. B3GALNT2 mutations associated with non-syndromic autosomal recessive intellectual disability reveal a lack of genotype-phenotype associations in the muscular dystrophy-dystroglycanopathies. Genome Med. 2017;9:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Al Dhaibani MA, El-Hattab AW, Ismayl O, Suleiman J. B3GALNT2-Related Dystroglycanopathy: Expansion of the Phenotype with Novel Mutation Associated with Muscle-Eye-Brain Disease, Walker-Warburg Syndrome, Epileptic Encephalopathy-West Syndrome, and Sensorineural Hearing Loss. Neuropediatrics. 2018;49:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |