Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7415
Peer-review started: September 24, 2021
First decision: March 3, 2022
Revised: April 8, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: July 26, 2022
Intravenous steroid pulse therapy is the treatment of choice for acute exacerbation of multiple sclerosis (MS). Although steroid administration is generally well-tolerated, cases of cardiac arrhythmia have been reported. Herein, we describe a young woman who developed marked sinus bradycardia and T-wave abnor
An 18-year-old woman experienced vertiginous dizziness and binocular diplopia 1 wk prior to admission. Neurological examination revealed left internuclear ophthalmoplegia with left peripheral-type facial palsy. The initial laboratory results were consistent with those of type 2 diabetes. Brain magnetic resonance imaging revealed multifocal, non-enhancing, symptomatic lesions and multiple enhancing lesions. She was diagnosed with MS and maturity-onset diabetes of the young. Intravenous methylprednisolone was administered. On day 5 after methylprednisolone infusion, marked bradycardia with T-wave abnormalities were observed. Genetic evaluation to elucidate the underlying conditions revealed a hepatocyte nuclear factor 4-alpha (HNF4A) gene mutation. Steroid treatment was discontinued under suspicion of corticosteroid-induced bradycardia. Her electrocardiogram changes returned to normal without complications two days after steroid discontinuation.
Corticosteroid-induced bradycardia may have a significant clinical impact, especially in patients with comorbidities, such as HNF4A mutations.
Core Tip: Corticosteroid administration may cause cardiac arrhythmias including severe bradycardia. The accompanying T-wave abnormalities may provide clues regarding the pathophysiology of corticosteroid-induced bradycardia. This case report highlights the need for clinical vigilance during high-dose corticosteroid administration, particularly in potentially vulnerable patients harboring genetic variants.
- Citation: Sohn SY, Kim SY, Joo IS. Corticosteroid-induced bradycardia in multiple sclerosis and maturity-onset diabetes of the young due to hepatocyte nuclear factor 4-alpha mutation: A case report. World J Clin Cases 2022; 10(21): 7415-7421
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7415.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7415
Multiple sclerosis (MS) is an immune-mediated demyelinating disorder of the central nervous system. Treatment strategies for MS can be divided into two categories: disease-modifying therapies (DMT) and treatment of acute relapses[1]. High-dose pulsed intravenous methylprednisolone infusion is the treatment of choice for acute exacerbation of MS, although other options such as oral methylprednisolone or plasma exchange can sometimes be considered[1]. Generally, there is no absolute contraindication for intravenous steroid pulse therapy, except for rare cases of documented hypersensitivity to methylprednisolone succinate or fulminant systemic fungal infections[2]. However, sinus bradycardia may occur because of steroid administration, especially in specific groups of patients with rheumatoid diseases[3-5], nephrotic syndrome[6], and MS[7].
Comorbid conditions may affect MS patients in terms of disease activity[8], quality of life, and treatment choice[9]. To date, the effects of comorbidities on treatment-related topics have been mainly investigated during DMT[10,11]. Herein, we describe a young woman who was diagnosed with MS and maturity-onset diabetes of the young (MODY) due to a hepatocyte nuclear factor 4-alpha (HNF4A) gene mutation. She developed marked sinus bradycardia and T-wave abnormalities during acute management with high-dose corticosteroids. Thus, the possible role of comorbid genetic disorders in the development of corticosteroid-induced bradycardia in this patient is explored.
An 18-year-old woman experienced vertiginous dizziness and binocular diplopia 1 wk before admission.
The patient’s symptoms started 1 wk ago. Diplopia was more pronounced in her right gaze. She was admitted to our hospital for evaluation of diabetes, which was confirmed at a local clinic. The patient was not overweight, and her body mass index was 20.5 kg/m2.
She was born by cesarean section at 36 6/7 wk. Her birthweight was 3800 g, which was significantly higher than that of her discordant twin sister, who weighed 2600 g. The patient was admitted to the Department of Pediatrics at the age of 3 and 5 years because of recurrent seizures with hypoglycemic events. Subsequent evaluations including brain magnetic resonance imaging (MRI) and extensive laboratory testing failed to ascertain the cause. However, she successfully attained developmental milestones during infancy and childhood.
Her paternal grandfather and maternal grandmother had diabetes. Other family members, including parents and twin sibling, did not have diabetes. She did not have a relevant family history of MS or other neurological disorders.
Neurological examination revealed an addction deficit of the left eye with abducting horizontal nystagmus of the right eye on attempting right gaze, which was compatible with left internuclear ophthalmoplegia. Mild left peripheral-type facial palsy (House-Brackmann grade I) was noted. Other cranial nerve functions; facial sensory examination; motor, sensory, and cerebellar function tests were unremarkable.
Subsequent laboratory test results were consistent with those of type 2 diabetes. Serum C-peptide level was normal, while insulin level was increased at 13.7 μIU (reference range: 1.1-11.6 μIU). Glycated hemoglobin was 10.4% (reference range: 4.3%-6.1%). Her anti-glutamic acid decarboxylase antibody levels were normal. Other laboratory evaluations, including anti-nuclear antibody, viral and parasite markers, anti-aquaporin 4 antibody, and anti-myelin oligodendrocyte glycoprotein antibody, were all negative. Cerebrospinal fluid-specific oligoclonal bands were identified by isoelectric focusing technique.
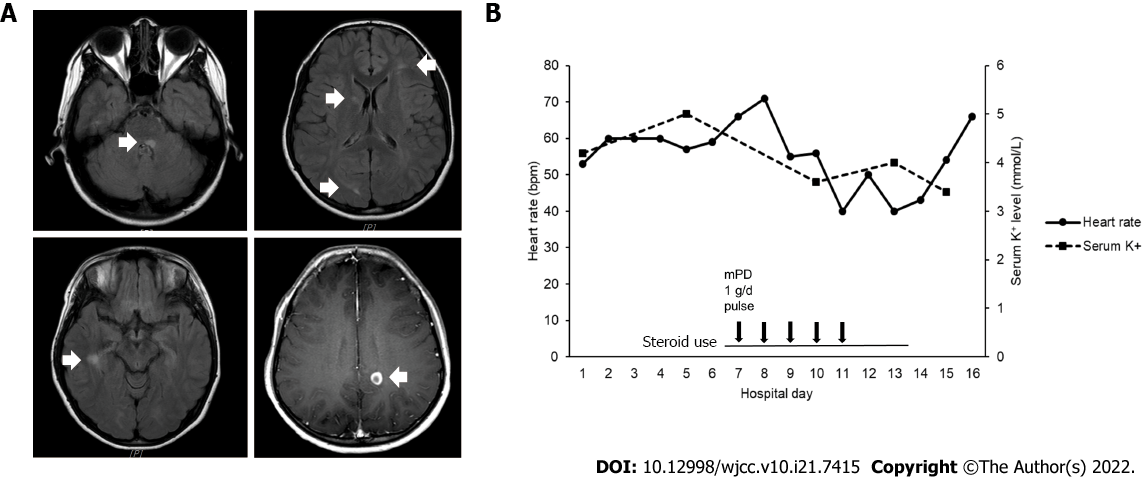
Brain MRI revealed a symptomatic lesion involving the left medial longitudinal fasciculus and facial colliculus in the posterior pons (Figure 1A), accompanied by multiple enhancing lesions in the left parietal, right occipital, and right temporal lobes.
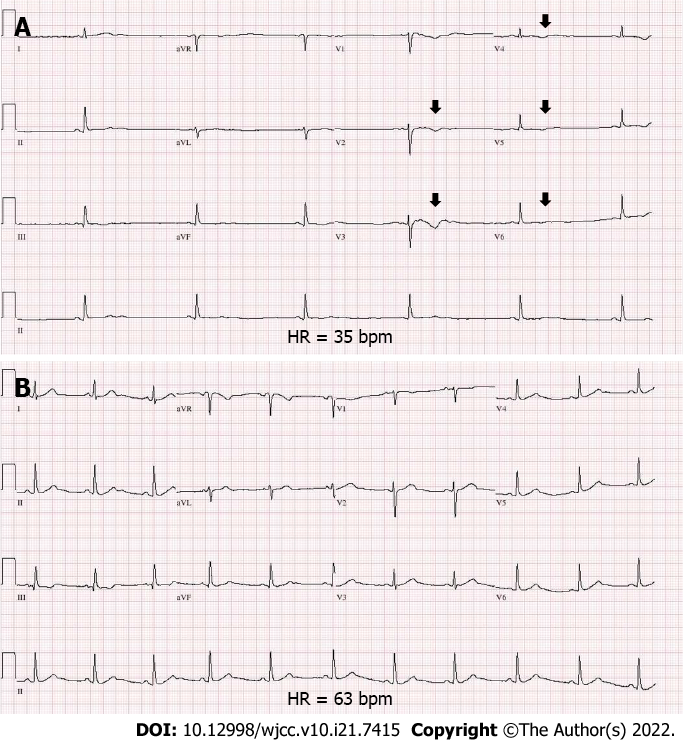
The diagnosis of MS was made based on the McDonald criteria 2017 revision. Intravenous methylprednisolone pulse therapy at a daily dosage of 1 g was initiated. On day 5 after methylprednisolone infusion, bradycardia was observed (Figure 1B). Electrocardiography (ECG) revealed marked sinus bradycardia with a heart rate (HR) of 35 beats per minute, T-wave flattening, and T-wave inversion (Figure 2A). The serum potassium level measured at the time of bradycardia was 4.0 mmol/L (reference range: 3.5-5.1 mmol/L) (Figure 1B). As steroid-induced bradycardia was suspected, oral prednisolone was discontinued. Echocardiography and Holter monitoring were unremarkable, except for sinus bradycardia. The cardiac enzyme levels were normal. Her ECG changes returned to baseline 2 days after steroid discontinuation (Figure 2B). Further evaluation was conducted to elucidate the underlying conditions. Diagnostic exome sequencing revealed a heterozygous variant of the HNF4A gene (NM_175914.4: c.1045C>T [p.Gln349*]). It was classified as a pathogenic variant according to the American College of Medical Genetics and Genomics guidelines[12], with evidence levels of PVS1 (null variant), PM2 (absent from population databases), and PP3 (deleterious in silico predictions). The same heterozygous mutation was reported in a study involving a large cohort of MODY[13].
The final diagnosis was MS, MODY, and corticosteroid-induced bradycardia with T-wave abnormalities associated with a HNF4A mutation.
She was treated with daily doses of dimethyl fumarate (480 mg), vitamin D (1000 IU), and metformin (1700 mg).
After 1 mo, the patient was neurologically free of symptoms. Follow-up imaging after 3 mo showed resolution of previous demyelinating lesions.
Loss of function of HNF4A is associated with MODY[14], congenital hyperinsulinemic hypoglycemia (HH)[13], and Fanconi renotubular syndrome[15]. MODY due to HNF4A mutation (formerly MODY1) is inherited in an autosomal dominant fashion[14,16]. HNF4A haploinsufficiency due to heterozygous mutations results in pancreatic β-cell dysfunction and impairs insulin secretion[16,17]. Although the patient’s parents did not have diabetes, her paternal grandfather and maternal grandmother did. The patient had been admitted to the Department of Pediatrics and had been evaluated for recurrent provoked seizure attacks with hypoglycemic events at the ages of 3 and 5 years (blood sugar level 0.8325 mmol/L and 1.4985 mmol/L, respectively). It is now evident that the HNF4A gene defect is the causative factor for these previous hypoglycemic events. Besides, HNF4A mutations are most common in exons 7 and 8 in the transactivation domain[14]. The nonsense variant in our patient was also predicted to be in a highly conserved position in exon 8.
Although corticosteroid-induced bradycardia was first reported in 1986[5], its precise pathological mechanism remains unclear. An early study by Fujimoto et al[6] showed that the fractional excretion of potassium and serum potassium significantly increased after methylprednisolone pulse therapy. The authors suspected that potassium efflux from the myocardial cells and the subsequent decrease in intracellular concentration might cause cardiac arrhythmia[6]. Other studies have suggested reflex bradycardia by activation of low-pressure baroreceptors or possible long QT syndrome gene mutations as possible mechanisms[4]. According to a recent literature review[7], corticosteroid-induced bradycardia seems to occur even with oral steroid administration, and a dose-dependent risk has also been reported[18]. In this patient, the HR decreased as corticosteroid was administered, and the baseline HR recovered after corticosteroid discontinuation. Regarding MS treatment, there was no choice but to quickly initiate DMT, since the use of high-dose corticosteroids—an essential therapy that helps alleviate acute flare-ups of MS[19]—should be avoided.
It is noteworthy that sinus bradycardia was accompanied by T-wave changes despite normokalemia in the present case. Flattening and inversion of T-waves have been known to be associated with hypokalemia. Patients with HNF4A mutations have defective proximal tubule function[20,21] and are prone to urinary loss of serum potassium[21]. Moreover, despite methylprednisolone generally having a minimal mineralocorticoid effect[22], serum potassium may decrease in response to a pulsed dose of methylprednisolone[23]. A sudden change in serum potassium levels in vulnerable patients may result in potassium efflux from myocardial cells[6] to maintain potassium homeostasis. Another explanation for T-wave morphological changes in the clinical setting of normokalemia is the possibility of a dysfunctional potassium channel[24]. In fact, HNF4A mutation is known to cause a reduction in the expression of the inwardly rectifying potassium (Kir) channel subunit 6.2 in pancreatic beta cells[25,26]. Although the role of HNF4A in the heart is largely unknown, there is some anecdotal evidence of HNF4a activity during cardiac development[27], and one can speculate that Kir channels may also show some degree of dysfunction in myocardial cells. Kir allows potassium ions to move easily into rather than out of the cell[28] and is expressed at high density in the cardiac sarcolemma[29]. Mineralocorticoid-induced hypertensive challenge in Kir 6.2 knockout mice resulted in atrioventricular conduction delay, heart failure, and bradycardia[29] which resembled corticosteroid-induced bradycardia. T-wave inversion after methylprednisolone pulse therapy seems to be extremely rare, according to a previous report[30]. An episode of cardiac arrhythmia with U waves on ECG after corticosteroid intake was previously reported in a case series study of Anderson-Tawil syndrome, a rare disease caused by defective Kir 2.1 protein due to KCNJ2 gene mutation[31].
The limitations of this study are briefly described below. Firstly, the genetic evaluation of other family members could not be performed because of non-medical issues. Secondly, the urinary excretion of potassium at the time of the cardiac event was not assessed. The patient’s HNF4A variant was not known during the admission period since the results of genetic testing only became available after 1 mo at the outpatient clinic. Thirdly, functional studies to confirm the arrhythmogenic effect of corticosteroids using Hnf4a knockout mouse models were not performed in this case. Lastly, the fact that this report is based on an observation of a single patient makes it difficult to generalize.
The potential genetic susceptibility for the development of drug-induced bradycardia has not been incorporated in recent guidelines[32]. To our knowledge, corticosteroid-induced bradycardia due to comorbid genetic disorders has not been previously described. We believe that our observations provide a direction for future research. One of the first steps would be to use mice models to explore the role of HNF4A mutation during corticosteroid administration. Systematic investigations are needed in the long run, to clarify the role of genetic comorbidities in patients with drug-induced bradyarrhythmia.
Corticosteroid-induced bradycardia may have a significant clinical impact, especially in MS patients with comorbid conditions, such as MODY due to HNF4A mutations. The present case is exceptional in that the T-wave abnormalities were accompanied by marked bradycardia. Considering that T-wave changes coincided with bradycardia events, abnormal potassium efflux or dysfunctional potassium channels due to HNF4A mutation might have contributed to the occurrence of corticosteroid-induced bradycardia. Patients with comorbid genetic mutations require special clinical attention, as in the present case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: MASARU T, Hungary; Zhao GH, China A-Editor: Liu X, China S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 952] [Cited by in F6Publishing: 1049] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 2. | Ocejo A, Correa R. Methylprednisolone. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2021, StatPearls Publishing LLC., 2021. [Cited in This Article: ] |
| 3. | Pudil R, Hrncir Z. Severe bradycardia after a methylprednisolone "minipulse" treatment. Arch Intern Med. 2001;161:1778-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Akikusa JD, Feldman BM, Gross GJ, Silverman ED, Schneider R. Sinus bradycardia after intravenous pulse methylprednisolone. Pediatrics. 2007;119:e778-e782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Tvede N, Nielsen LP, Andersen V. Bradycardia after high-dose intravenous methylprednisolone therapy. Scand J Rheumatol. 1986;15:302-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Fujimoto S, Kondoh H, Yamamoto Y, Hisanaga S, Tanaka K. Holter electrocardiogram monitoring in nephrotic patients during methylprednisolone pulse therapy. Am J Nephrol. 1990;10:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Stroeder J, Evans C, Mansell H. Corticosteroid-induced bradycardia: Case report and review of the literature. Can Pharm J (Ott). 2015;148:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Salter A, Kowalec K, Fitzgerald KC, Cutter G, Marrie RA. Comorbidity is associated with disease activity in MS: Findings from the CombiRx trial. Neurology. 2020;95:e446-e456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Magyari M, Sorensen PS. Comorbidity in Multiple Sclerosis. Front Neurol. 2020;11:851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Zhang T, Tremlett H, Leung S, Zhu F, Kingwell E, Fisk JD, Bhan V, Campbell TL, Stadnyk K, Yu BN, Marrie RA; CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology. 2016;86:1287-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Laroni A, Signori A, Maniscalco GT, Lanzillo R, Russo CV, Binello E, Lo Fermo S, Repice A, Annovazzi P, Bonavita S, Clerico M, Baroncini D, Prosperini L, La Gioia S, Rossi S, Cocco E, Frau J, Torri Clerici V, Signoriello E, Sartori A, Zarbo IR, Rasia S, Cordioli C, Cerqua R, Di Sapio A, Lavorgna L, Pontecorvo S, Barrilà C, Saccà F, Frigeni B, Esposito S, Ippolito D, Gallo F, Sormani MP; iMUST group. Assessing association of comorbidities with treatment choice and persistence in MS: A real-life multicenter study. Neurology. 2017;89:2222-2229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13374] [Cited by in F6Publishing: 17935] [Article Influence: 1992.8] [Reference Citation Analysis (0)] |
| 13. | Flanagan SE, Kapoor RR, Mali G, Cody D, Murphy N, Schwahn B, Siahanidou T, Banerjee I, Akcay T, Rubio-Cabezas O, Shield JP, Hussain K, Ellard S. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol. 2010;162:987-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013;34:669-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Clemente M, Vargas A, Ariceta G, Martínez R, Campos A, Yeste D. Hyperinsulinaemic hypoglycaemia, renal Fanconi syndrome and liver disease due to a mutation in the HNF4A gene. Endocrinol Diabetes Metab Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ferrer J. A genetic switch in pancreatic beta-cells: implications for differentiation and haploinsufficiency. Diabetes. 2002;51:2355-2362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Marucci A, Rutigliano I, Fini G, Pezzilli S, Menzaghi C, Di Paola R, Trischitta V. Role of Actionable Genes in Pursuing a True Approach of Precision Medicine in Monogenic Diabetes. Genes (Basel). 2022;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Üsküdar Cansu D, Bodakçi E, Korkmaz C. Dose-dependent bradycardia as a rare side effect of corticosteroids: a case report and review of the literature. Rheumatol Int. 2018;38:2337-2343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Tanaka M, Vécsei L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Hamilton AJ, Bingham C, McDonald TJ, Cook PR, Caswell RC, Weedon MN, Oram RA, Shields BM, Shepherd M, Inward CD, Hamilton-Shield JP, Kohlhase J, Ellard S, Hattersley AT. The HNF4A R76W mutation causes atypical dominant Fanconi syndrome in addition to a β cell phenotype. J Med Genet. 2014;51:165-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Anyiam O, Wallin E, Kaplan F, Lawrence C. A Complicated Pregnancy in an Adult with HNF4A p.R63W-Associated Fanconi Syndrome. Case Rep Med. 2019;2019:2349470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D. Immune Effects of Corticosteroids in Sepsis. Front Immunol. 2018;9:1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Yong KL, Chng CL, Htoon HM, Lim LH, Seah LL. Safety Profile and Effects of Pulsed Methylprednisolone on Vital Signs in Thyroid Eye Disease. Int J Endocrinol. 2015;2015:457123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 25. | Qi L, van Dam RM, Asselbergs FW, Hu FB. Gene-gene interactions between HNF4A and KCNJ11 in predicting Type 2 diabetes in women. Diabet Med. 2007;24:1187-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Harris AP, Ismail KA, Nunez M, Martopullo I, Lencinas A, Selmin OI, Runyan RB. Trichloroethylene perturbs HNF4a expression and activity in the developing chick heart. Toxicol Lett. 2018;285:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1009] [Cited by in F6Publishing: 1055] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 29. | Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285-2297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Rottensteiner J, Kaneppele A, Stockner I, Ladurner C, Panizza G, Wiedermann CJ. Precordial T-wave inversion of "cardiac memory" pattern after high-dose methylprednisolone pulse therapy. Intern Emerg Med. 2008;3:375-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Bendahhou S, Fournier E, Gallet S, Ménard D, Larroque MM, Barhanin J. Corticosteroid-exacerbated symptoms in an Andersen's syndrome kindred. Hum Mol Genet. 2007;16:900-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Tisdale JE, Chung MK, Campbell KB, Hammadah M, Joglar JA, Leclerc J, Rajagopalan B; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Drug-Induced Arrhythmias: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e214-e233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |