Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5723
Peer-review started: October 23, 2021
First decision: December 17, 2021
Revised: December 20, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Ectopic adrenocorticotropic hormone (ACTH)-secreting neuroendocrine tumors are rare diseases. Patients with ACTH-secreting pancreatic neuroendocrine carcinomas have a poor prognosis. Infections and coagulopathies have been reported as the cause of death. However, detailed clinical descriptions of the morbid complications of ACTH-secreting neuroendocrine carcinomas have not been reported.
A 78-year-old Japanese woman consulted a medical center due to systemic edema and epigastric discomfort. Laboratory analysis revealed hypercortisolemia with increased ACTH secretion without diurnal variation in serum cortisol level. An enhanced computed tomography (CT) scan revealed a 3-cm tumor in the pancreatic head. The cytological material from endoscopic ultrasound-guided fine-needle aspiration was compatible with ACTH-secreting pancreatic neuroendocrine carcinoma. The Ki-67 index was 40%. She was transferred to Mie University Hospital for surgical treatment. The patient was diagnosed with urinary tract infection, cytomegalovirus hepatitis, esophageal candidiasis, pulmonary infiltrates suspicious for Pneumocystis carinii pneumonia, peripheral deep vein thrombosis, pulmonary embolism, and disseminated intravascular coagulation. The multiple organ infections and thromboses responded well to antimicrobial and anticoagulant therapy. Radioisotope studies disclosed a pancreatic tumor and a metastatic lesion in the liver, whereas somatostatin receptor scintigraphy showed negative findings, suggesting the primary and metastatic tumors were poorly differentiated. A CT scan before admission showed no metastatic liver lesion, suggesting that the pancreatic tumor was rapidly progressing. Instead of surgery, antitumor chemotherapy was indicated. The patient was transferred to another hospital to initiate chemotherapy. However, she died four months later due to the rapidly progressive tumor.
ACTH-secreting pancreatic neuroendocrine neoplasm is a rare disease with a very poor prognosis. The clinical course and acute complications of the tumor remain unreported. Here we report the clinical course of a rapidly progressive case of ACTH-secreting pancreatic neuroendocrine tumor that developed infectious complications due to many types of pathogens in multiple organs, widespread thromboses, pulmonary embolism, and disseminated intravascular coagulation.
Core Tip: Adrenocorticotropic hormone (ACTH)-secreting pancreatic neuroendocrine tumor is a rare malignant disease with a poor prognosis. The condition is frequently associated with infectious and thrombotic complications. However, the detailed clinical course and acute complications of the tumor remain unreported. Herein, we report a rare case of ACTH-secreting pancreatic neuroendocrine tumor associated with infections due to multiple pathogens in several organs and systemic coagulopathies. The infectious and thrombotic complications responded well to antibiotics, antiviral and antifungal drugs, and anticoagulants. However, radioisotope studies showed that the tumor was poorly differentiated, rapidly progressive with multiple metastatic lesions in the liver. On this basis, instead of surgical treatment, antitumor chemotherapy was indicated. Unfortunately, the patient died due to systemic tumor dissemination.
- Citation: Yoshihara A, Nishihama K, Inoue C, Okano Y, Eguchi K, Tanaka S, Maki K, Fridman D'Alessandro V, Takeshita A, Yasuma T, Uemura M, Suzuki T, Gabazza EC, Yano Y. Adrenocorticotropic hormone-secreting pancreatic neuroendocrine carcinoma with multiple organ infections and widespread thrombosis: A case report. World J Clin Cases 2022; 10(17): 5723-5731
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5723.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5723
Neuroendocrine tumors or neuroendocrine neoplasms are classified by the World Health Organization into "well-differentiated" and "poorly differentiated" neuroendocrine carcinomas[1]. Ectopic adrenocorticotropic hormone (ACTH)-secreting neuroendocrine tumors account for 9%-18% of Cushing's syndrome cases[2]. Among ectopic ACTH-secreting tumors, pancreatic neuroendocrine (pNE) tumors are rare (4%-16%) but very aggressive tumors[3]. Previous studies have shown that patients with ACTH-secreting pNE neoplasms have a poorer prognosis than patients with insulinoma, gastrinoma, and non-functioning pNE tumors[4]. In addition, pneumocystis pneumonia, pulmonary embolism, and other complications have been reported as causes of death in patients with ACTH-secreting pNE tumors[5-8]. However, previous reports provided no detailed clinical descriptions of the morbid complications of ACTH-secreting pNE tumors. Clinical follow-up with studies using radioisotope scans is also lacking. Here we report the detailed clinical course of a rapidly progressive case of ACTH-secreting pNE tumor that developed several complications, including multiple organ infections due to many types of pathogens, disseminated intravascular coagulation, and pulmonary thromboembolism. This case study shows that the progressive and aggressive nature of ACTH-secreting pNE tumors gives no truce for treating the disease with more effective therapeutic modalities, including surgical resection.
The chief complaints of the patient were systemic edema and epigastric discomfort.
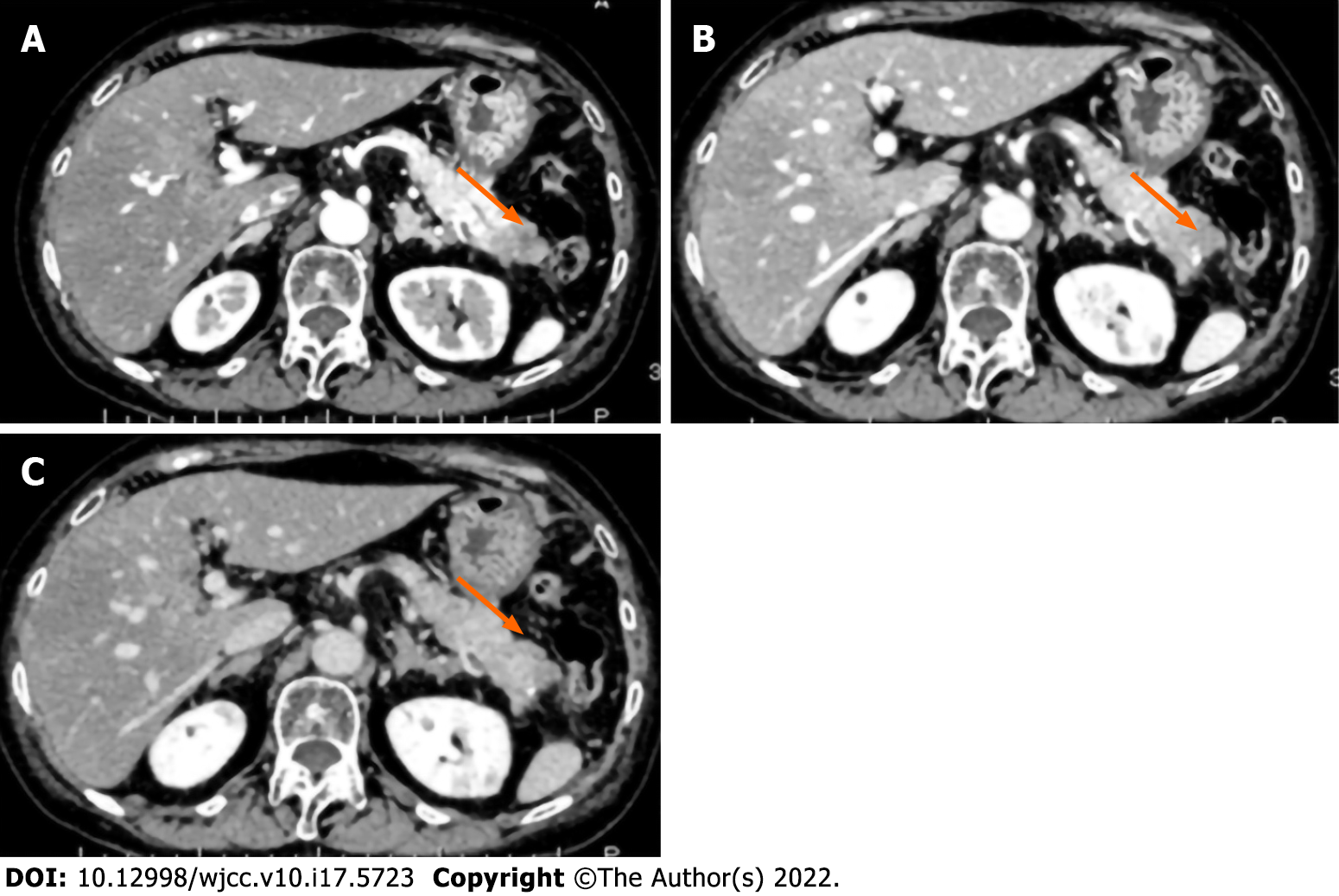
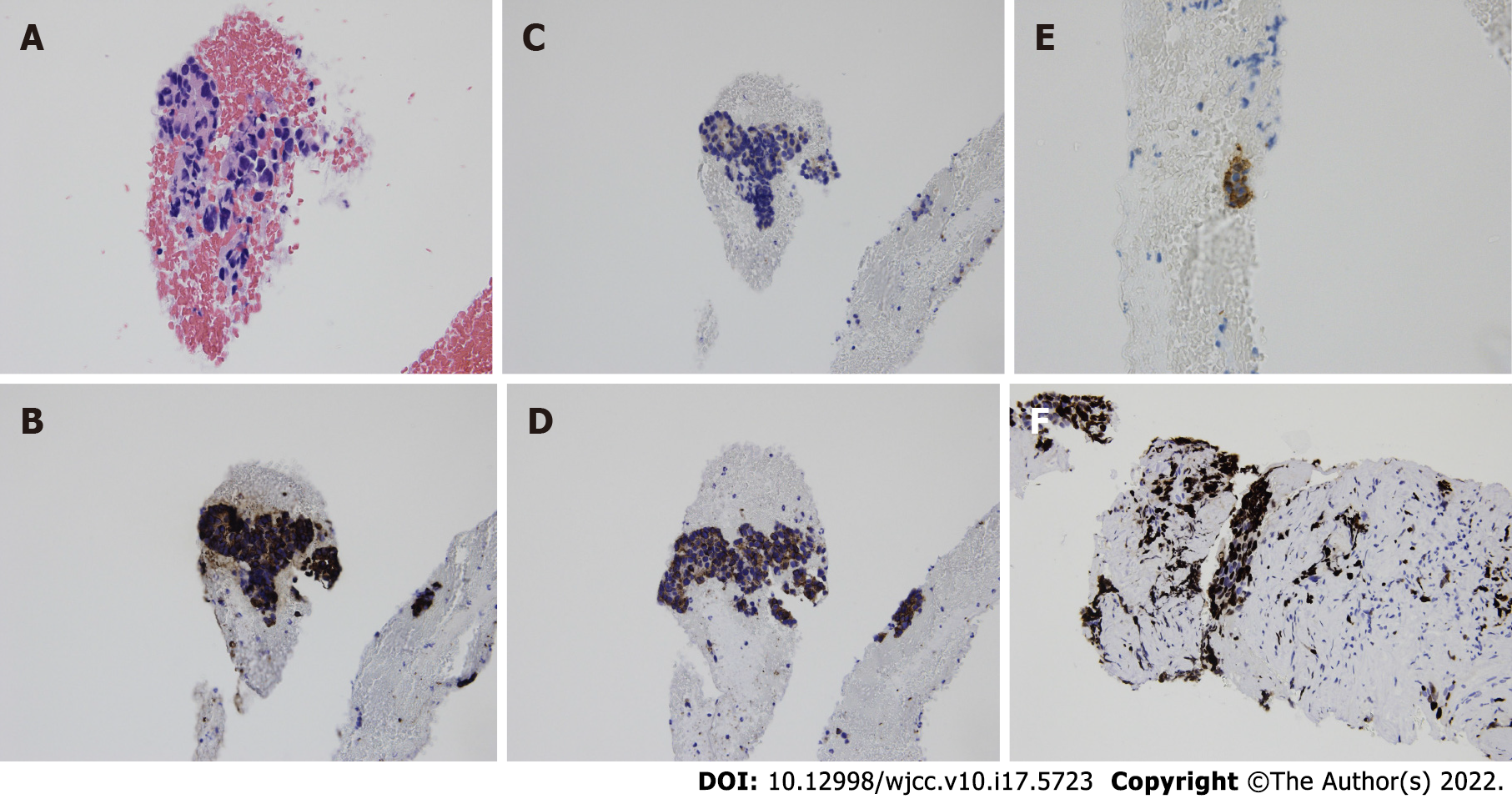
Two months before admission to our institution, a 78-year-old Japanese woman consulted a medical center due to systemic edema and epigastric discomfort. Laboratory analysis showed no abnormalities. Therefore, close clinical follow-up was suggested. However, blood analysis performed two months later revealed a high hemoglobin A1c (5.5%-7.2%) and decreased potassium concentration (2.7 mEq/L). Screening of potential causes of secondary diabetes mellitus showed high levels of cortisol (80.6 μg/dL) and ACTH (177 pg/mL) in the early morning and increased levels of cortisol (56.2 μg/dL) at midnight. The daily urine cortisol level (6550 μg/day) was also increased. A dexamethasone suppressive test (8 mg) showed no suppression of fasting blood cortisol (118 μg/dL) or ACTH (342 pg/mL). These results suggested the diagnosis of ACTH-dependent hypercortisolism (Table 1). An abdominal computed tomography (CT) scan showed a 3-cm tumor with poor contrast in the pancreatic tail (Figure 1). Cytological findings of endoscopic ultrasound-guided fine-needle aspiration showed cell clusters forming no specific structures with a high nuclear/cytoplasmic ratio, suggesting adenocarcinoma (Figure 2A). However, the immunostaining results showed positivity for chromogranin A, synaptophysin, CD 56, and ACTH (Figure 2B-E) that were compatible with the diagnosis of ACTH-secreting pNE neoplasm. The Ki-67 index was approximately 40% (Figure 2F). The patient was then referred to Mie University Hospital for subsequent examinations and treatment.
| Blood cell count | RR | Units | Biochemical and immunology | RR | Units | Endocrinology | RR | Units | |||
| White blood cell | 6530 | 3300-8600 | /μL | Total protein | 4.7 | 6.6-8.1 | g/dL | Cortisol | 134.2 | 4.5-21.1 | μg/dL |
| Neutrophils | 91.1 | 37.0-72.0 | % | Albumin | 2.8 | 4.1-5.1 | g/dL | Cortisol (23:00)1 | 56.2 | μg/dL | |
| Lymphocytes | 6.0 | 20.0-50.9 | % | BUN | 21.3 | 8.0-20.0 | mg/dL | Cortisol (8 mg-DST)1 | 118.0 | μg/dL | |
| CD3+CD4+ | 24.06 | 40.4-57.4 | % | Creatinine | 0.67 | 0.46-0.79 | mg/dL | Urine cortisol1 | 6,550 | 26-187 | μg/day |
| CD4+CD8+ | 15.46 | 15.0-30.0 | % | Uric acid | 2.2 | 2.6-5.5 | mg/dL | ACTH | 134.2 | 4.5-21.1 | pg/mL |
| CD4+/CD8+ ratio | 1.56 | Na | 150 | 138-145 | mEq/L | ACTH (8 mg-DST)1 | 342.0 | pg/mL | |||
| Monocytes | 2.6 | 4.1-10.6 | % | K | 2.7 | 3.6-4.8 | mEq/L | ||||
| Eosinophils | 0.0 | 0.6-8.3 | % | Cl | 108 | 101-108 | mEq/L | Tumor Marker | RR | Units | |
| Basophils | 0.3 | 0.0-1.3 | % | Ca | 7.9 | 8.8-10.1 | mg/dL | CEA | 4.8 | < 5.2 | ng/mL |
| Red blood cell | 489 | 386-492 | × 104/μL | P | 2.2 | 2.7-4.6 | mg/dL | CA19-9 | 28.8 | < 36.8 | U/mL |
| Hemoglobin | 12.5 | 11.6-14.8 | g/dL | AST | 121 | 13-30 | U/L | DUPAN-2 | 47 | 0-150 | U/mL |
| Hematocrit | 37.3 | 35.1-44.1 | % | ALT | 294 | 7-23 | U/L | SPAN-1 | 15 | 0-30 | U/mL |
| Platelet | 37 | 15.8-34.8 | × 104/μL | LDH | 978 | 124-222 | U/L | ||||
| γ-GTP | 226 | 9-32 | U/L | Infection | RR | Units | |||||
| Coagulation | RR | Units | ALP | 514 | 106-322 | U/L | HBsAg | < 0.01 | < 0.05 | IU/mL | |
| APTT | 20.7 | < 37.0 | Seconds | T-Bil | 1.0 | 0.4-1.5 | mg/dL | HBsAb | 0.37 | < 10.00 | IU/mL |
| PT | 11.1 | 9.8-12.1 | Seconds | CRP | 0.26 | < 0.14 | mg/dL | HBcAb | 0.03 | < 1.00 | S/CO |
| D-dimer | 30.19 | < 1.00 | μg/mL | Total-cholesterol | 209 | 142-248 | mg/dL | HCVAb | 0.05 | < 1.00 | S/CO |
| FDP | 80.1 | < 5.0 | μg/mL | Triglyceride | 129 | 30-117 | mg/dL | β-D Glucan | 9.2 | < 11.0 | pg/mL |
| HbA1c | 7.8 | 4.9-6.0 | % | CMV-antigen (C7-HRP) | 132/82500 | cells | |||||
| Plasma glucose | 246 | 73-109 | mg/dL | ||||||||
| IgG | 498 | 861-1747 | mg/dL | ||||||||
| IgM | 169 | 93-393 | mg/dL | ||||||||
| IgM | 104 | 50-269 | mg/dL | ||||||||
There was no remarkable past or family history. She had no history of allergy, excessive alcohol consumption, or smoking.
The clinical findings on admission to Mie University Hospital were as follows: height 157.5 cm, body weight 54 kg, blood pressure 135/79 mmHg, and pulse rate 91 bpm. In addition, the patient presented a "moon-face" with bilateral pitting edema on the lower limbs on physical examination.
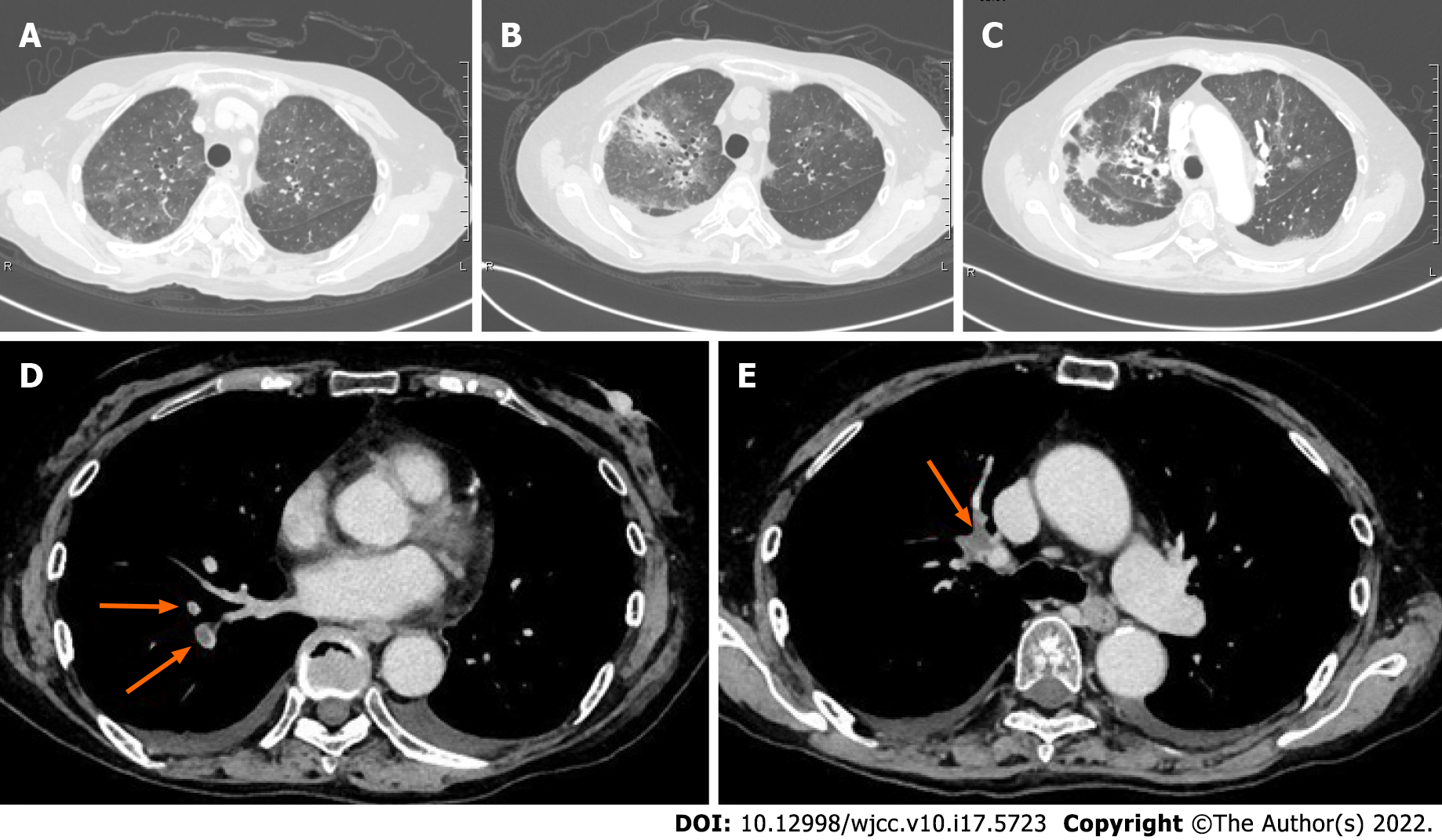
Laboratory analysis revealed thrombocytopenia with elevated blood levels of D-dimer, fibrinogen/fibrin degradation products, liver enzymes, cortisol and ACTH, and hypokalemia (Table 1). Brain magnetic resonance imaging (MRI) showed no pituitary tumor. Blood culture was negative, but urine culture was positive for Escherichia coli and Pseudomonas aeruginosa. Esophageal candidiasis was diagnosed by esophagogastroduodenoscopy. The patient was also positive for Cytomegalovirus antigen (C7-HRP, on day 1, 132/82500 cells) associated with increased levels of liver enzymes (AST 121 U/L, ALT 294 U/L on day 1), and thus Cytomegalovirus hepatitis was diagnosed. Additional laboratory analysis on day 7 revealed an increased level of β-D glucan (48.0 pg/mL), and a chest CT scan on day 9 showed bilateral ground-glass opacity (Figure 3A and B) suspicious for Pneumocystis pneumonia.
In addition to laboratory findings of disseminated intravascular coagulation, venous ultrasonography of the lower extremities performed on day 1 revealed deep venous thrombosis in the right popliteal segment of the femoral vein (size not recorded), right soleus vein (5-6 cm), and left posterior tibial vein (4 cm). In addition, pulmonary embolism was pointed out by a radiologist based on a chest CT scan taken on day 9 (Figure 3D and E).
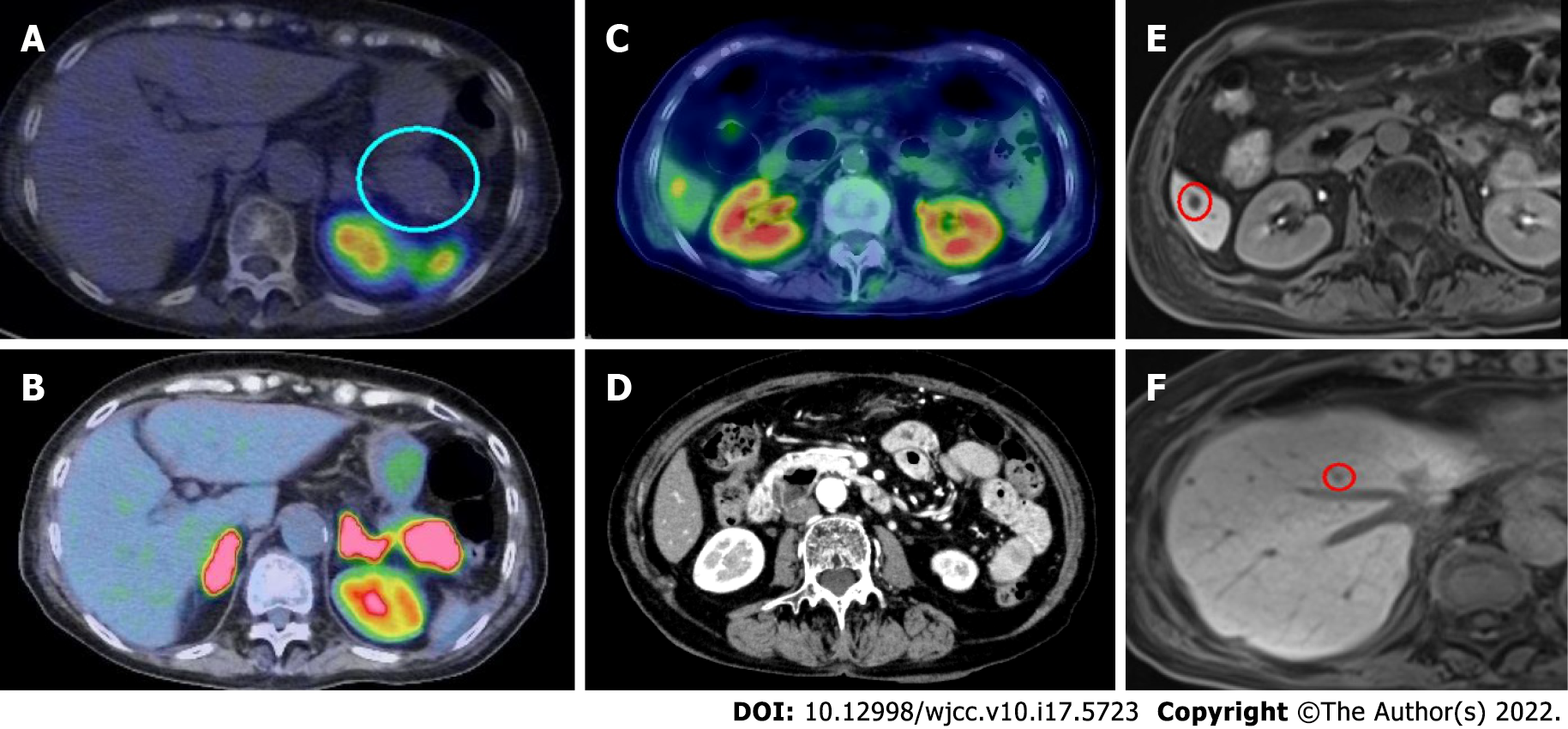
Somatostatin receptor scintigraphy with 111In-pentetoreotide performed on day 21 showed no uptake in any organ (Figure 4A). However, an 18F-fluorodeoxyglucose-positron emission tomography (PET) performed on day 29 showed positive uptake in the pancreatic tumor and the liver (S6 segment; Figure 4B and C). Enhanced CT imaging taken before transfer to Mie University Hospital showed no tumor in the liver S6 segment (Figure 4D). Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-MRI revealed multiple liver tumors suggesting liver metastases (Figure 4E and F). Based on these findings, surgical intervention was canceled.
The patient's final diagnosis was ACTH-secreting pNE tumor with ACTH-dependent hypercortisolism associated with urinary tract infection, Cytomegalovirus hepatitis, esophageal candidiasis, pulmonary infiltrates suspicious for Pneumocystis carinii pneumonia, and widespread peripheral deep vein thromboses, pulmonary embolism, and disseminated intravascular coagulation.
The pancreas tumor-related hypercortisolism and hypokalemia were treated with metyrapone (1500 to 3000 mg/day), hydrocortisone (60 mg/day), potassium chloride (2400-3600 mg/day) and spironolactone (50 mg/day). Hyperglycemia was treated with multiple daily insulin injections (up to 25 U/day, days 1 to 48) and oral repaglinide (0.75 mg/day). The response to therapy was good with a postprandial blood glucose level of 172 mg/dL, and HbA1c of 5.5% on day 88.
The urinary tract infection was treated with meropenem (2 g/day, days 2 to 5) and ceftazidime (2 g/day, days 6 to 15). Esophageal candidiasis was treated with oral fluconazole (100 mg/day, from days 1 to 15). Cytomegalovirus hepatitis was empirically treated with foscarnet (5 g/day) from day 5 after admission. Treatment with foscarnet was stopped on day 27 because the liver enzymes improved (AST 32 U/L, ALT 66 U/L on day 9), and the C7-HRP test was negative.
The pulmonary infiltrates (Figure 3A and B) suspicious for Pneumocystis pneumoniae were treated with oral trimethoprim-sulfamethoxazole (480-2400 mg/day) for 3 wk. The β-D glucan level (10.4 pg/mL) decreased, and the respiratory status was stable on day 27. Prednisolone (25 mg/day) was added to the treatment because another radiological study disclosed organizing pneumonia on day 29 (Figure 3C). Treatment with oral trimethoprim-sulfamethoxazole (80-400 mg/day, three times a week) was also continued.
The patient's hypercoagulable states and thrombotic complications were treated with nafamostat mesylate (200 mg/day, days 1 to 9) and gabexate mesylate (1500 mg/day, days 10 to19). Due to the presence of severe thrombocytopenia, the patient received 10 units of platelet transfusion on day 6. A radiologist pointed out pulmonary embolism based on a chest CT scan taken on day 9 (Figure 3D and E). Treatment with apixaban was started from day 11 (20 mg/day for the first 7 days, 10 mg/day thereafter). Disseminated intravascular coagulation improved, and the thrombi in the pulmonary artery and deep veins completely disappeared on day 88.
The multiple organ infections and thromboses responded well to antimicrobial and anticoagulant therapy. However, a radioisotope study with PET disclosed a pancreatic tumor and a metastatic lesion in the liver, whereas somatostatin receptor scintigraphy showed negative findings, suggesting that the primary and metastatic tumors were poorly differentiated and rapidly progressive. Based on these findings, surgical intervention was canceled. The patient was then transferred to another institution for antitumor chemotherapy. However, she died four months later due to rapid tumor progression.
ACTH-secreting pNE tumors are rare malignant diseases with aggressive behavior[3]. Patients with this malignant disease have a poorer prognosis than patients with insulinoma, gastrinoma, or non-functioning ACTH-secreting pNE tumors[4]. However, the explanation for the tumor aggressiveness and the poor patient prognosis remains unclear. A previous study suggested that hypomethylation of the proopiomelanocortin promoter may potentiate the ACTH secretory property of the tumors[8]. A high Ki-67 index indicates poor clinical outcomes in pNE tumors[1,9]. The Ki-67 index was high in our present case. A pathological diagnosis of a poorly differentiated pNE tumor also predicts a poor prognosis[1]. Cytological study of samples taken by needle aspiration was available in our patient. However, it was difficult to determine the tumor differentiation status based only on the cytological material. Previous reports suggested that radioisotope techniques may help determine cell differentiation status.
In addition to allowing localization of the primary malignant tumor and metastatic lesion, radioisotope studies of pNE tumors may provide information on the tumor cell metabolic activity and the expression of specific receptors such as somatostatin receptors[10]. In the current case, we performed somatostatin receptor scintigraphy using 111In-pentetoreotide and PET using 18F-fluorodeoxyglucose. The PET study confirmed the primary pNE tumor and the presence of liver metastasis, whereas the scintigraphy disclosed no tumor uptake. The combination of positive 18F-fluorodeoxyglucose uptake by PET and negative 111In-pentetoreotide uptake by scintigraphy indicates poor differentiation of pNE tumor cells[11,12]. Malcolm et al[10] reported a sensitivity of 57% for somatostatin receptor scintigraphy using 111In-pentetoreotide and 100% for 18F-fluorodeoxyglucose-PET in Grade 3 pNE tumors. Another study showed that metastasis of highly differentiated pNE tumors can be detected by somatostatin receptor scintigraphy using 111In-pentetoreotide but not by 18F-fluorodeoxyglucose-PET[13]. In the present case, the 18F-fluorodeoxyglucose-PET study showed positive radioisotope uptake in the primary pNE tumor and metastatic liver lesion, but the scintigraphy showed no radioisotope uptake, suggesting that the primary and metastatic pNE tumor was not differentiated.
In the current case, the ACTH-secreting pNE tumor was associated with multiple organ infections, including urinary tract infection, viral hepatitis, esophageal candidiasis, and bilateral pulmonary infiltrates suspicious for Pneumocystis carinii pneumonia. Patients with hypercortisolemia are immunocompromised[14]. Immunosuppression in patients with hypercortisolism is generally characterized by the reduced bactericidal function of neutrophils, decreased CD14- and CD16-mediated phagocytic activity of monocytes, deficient cytotoxic activity of natural killer cells, and reduced number of CD4+ T cells[14]. The current case showed a reduction in lymphocytes, including CD4+ cells, and decreased peripheral blood concentration of IgG, suggesting she was an immunocompromised host. However, infections with multiple microbes responded well to antibacterial, antifungal, and antiviral drugs. The diagnosis of Pneumocystis carinii pneumonia in our patient was not confirmed by microscopic observation of P. jirovecii or positivity by polymerase chain reaction. However, we initiated therapy for Pneumocystis carinii pneumonia after suspecting the disease based on lung CT findings as delayed treatment of Pneumocystis carinii pneumonia may be fatal in patients with ectopic ACTH syndrome[5,15,16].
In the current case, the ACTH-secreting pNE tumor was also associated with many thrombotic complications, including thrombus formation in many limb peripheral veins, pulmonary embolism, and disseminated intravascular coagulation. Patients with hypercortisolemia are in a hypercoagulable state[17]. Hypercortisolism may cause hypercoagulability, although the precise mechanism is unclear. Alterations in the blood levels of von Willebrand factor and factor VIII have been reported[17]. Some polymorphisms appear to affect the promoter activity of the von Willebrand factor gene in response to glucocorticoids, and a high concentration of cortisol may alter the multimeric structure of von Willebrand factor[17]. Decreased physical activity during hospitalization or the perioperative period is also a contributing factor to increased risk of venous thrombosis and pulmonary embolism in patients with hypercortisolemia[18,19]. The risk of thrombosis was high in our patient because of reduced physical activity and the presence of malignancy and infectious diseases[20,21]. Our patient had a decreased number of platelets on admission. We delayed the treatment with anticoagulant therapy to prevent fatal hemorrhage. However, earlier anticoagulation therapy would have improved the thrombotic complications in the patient.
This report described the clinical course of a rapidly progressive case of ACTH-secreting pNE tumor who developed infectious complications due to many types of pathogens in multiple organs, widespread thromboses, pulmonary embolism, and disseminated intravascular coagulation. This case study shows that neuroendocrine carcinoma of the pancreas secreting ACTH has a poor immune state and that the progressive and aggressive nature of ACTH-secreting pNE tumors gives no truce for treating the disease with more effective therapeutic modalities, including surgical resection.
The authors would like to thank Dr. Tetsuya Murata from the JA Suzuka General Hospital Division of Pathology.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anoshkin K, Russia; Hu HG, China; Sharaf M, Syria S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1833] [Cited by in F6Publishing: 1677] [Article Influence: 419.3] [Reference Citation Analysis (2)] |
| 2. | Wajchenberg BL, Mendonca BB, Liberman B, Pereira MA, Carneiro PC, Wakamatsu A, Kirschner MA. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994;15:752-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 851] [Cited by in F6Publishing: 858] [Article Influence: 107.3] [Reference Citation Analysis (1)] |
| 4. | Maragliano R, Vanoli A, Albarello L, Milione M, Basturk O, Klimstra DS, Wachtel A, Uccella S, Vicari E, Milesi M, Davì MV, Scarpa A, Sessa F, Capella C, La Rosa S. ACTH-secreting pancreatic neoplasms associated with Cushing syndrome: clinicopathologic study of 11 cases and review of the literature. Am J Surg Pathol. 2015;39:374-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Cristante J, Lepelley M, Mallaret M, Carreau A, Chabre O. Pneumocystis pneumonia can complicate medical treatment of hypercortisolism even in outpatients with Cushing's disease. Ann Endocrinol (Paris). 2020;81:551-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Schernthaner-Reiter MH, Siess C, Micko A, Zauner C, Wolfsberger S, Scheuba C, Riss P, Knosp E, Kautzky-Willer A, Luger A, Vila G. Acute and Life-threatening Complications in Cushing Syndrome: Prevalence, Predictors, and Mortality. J Clin Endocrinol Metab. 2021;106:e2035-e2046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Shayesteh S, Fouladi DF, Fishman EK, Kawamoto S. Ectopic Cushing syndrome caused by a pancreatic neuroendocrine tumor: A case report. Radiol Case Rep. 2020;15:1014-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zhang C, Jin J, Xie J, Ye L, Su T, Jiang L, Zhou W, Jiang Y, Wu L, Wang T, Zhong X, Ning G, Shen B, Wang W. The Clinical Features and Molecular Mechanisms of ACTH-secreting Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Hamilton NA, Liu TC, Cavatiao A, Mawad K, Chen L, Strasberg SS, Linehan DC, Cao D, Hawkins WG. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152:107-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Squires MH 3rd, Volkan Adsay N, Schuster DM, Russell MC, Cardona K, Delman KA, Winer JH, Altinel D, Sarmiento JM, El-Rayes B, Hawk N, Staley CA 3rd, Maithel SK, Kooby DA. Octreoscan Versus FDG-PET for Neuroendocrine Tumor Staging: A Biological Approach. Ann Surg Oncol. 2015;22:2295-2301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A, Barnes T, Diakos C, Khasraw M, Samra J, Eslick E, Roach PJ, Engel A, Clarke SJ, Bailey DL. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics. 2017;7:1149-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Kubota K, Okasaki M, Minamimoto R, Miyata Y, Morooka M, Nakajima K, Sato T. Lesion-based analysis of (18)F-FDG uptake and (111)In-Pentetreotide uptake by neuroendocrine tumors. Ann Nucl Med. 2014;28:1004-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Yasuda M, Takeda S, Lee M, Hoshi S, Hoshi T, Tanaka Y, Miyajima S, Takaya H, Kajimura K. Small Cystic Pancreatic Neuroendocrine Neoplasm with Huge Liver and Bone Metastases. Intern Med. 2020;59:3027-3032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The Immune System in Cushing's Syndrome. Trends Endocrinol Metab. 2020;31:655-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Colby C, McAfee S, Sackstein R, Finkelstein D, Fishman J, Spitzer T. A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;24:897-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Song Y, Ren Y, Wang X, Li R. Recent Advances in the Diagnosis of Pneumocystis Pneumonia. Med Mycol J. 2016;57:E111-E116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Trementino L, Arnaldi G, Appolloni G, Daidone V, Scaroni C, Casonato A, Boscaro M. Coagulopathy in Cushing's syndrome. Neuroendocrinology. 2010;92 Suppl 1:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Suarez MG, Stack M, Hinojosa-Amaya JM, Mitchell MD, Varlamov EV, Yedinak CG, Cetas JS, Sheppard B, Fleseriu M. Hypercoagulability in Cushing Syndrome, Prevalence of Thrombotic Events: A Large, Single-Center, Retrospective Study. J Endocr Soc. 2020;4:bvz033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Wagner J, Langlois F, Lim DST, McCartney S, Fleseriu M. Hypercoagulability and Risk of Venous Thromboembolic Events in Endogenous Cushing's Syndrome: A Systematic Meta-Analysis. Front Endocrinol (Lausanne). 2018;9:805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel). 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 21. | Mullard A, Innes H. Venous thromboembolism in malignancy. Clin Med (Lond). 2014;14:532-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |