Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3923
Peer-review started: September 21, 2021
First decision: December 27, 2021
Revised: February 3, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Streptococcus mitis (S. mitis) is an opportunistic pathogen that can lead to severe ocular infections. In previous reports, penetrating keratoplasty (PK) was usually adopted for the treatment of persistent corneal ulcers. This report describes an unusual case of nonhealing descemetocele caused by S. mitis treated by antibiotics plus amniotic membrane transplantation (AMT).
A 63-year-old woman presented with a right persistent corneal ulcer that she had suffered from for the past 9 mo. The culture of a corneal scraping yielded S. mitis. The right eye descemetocele decreased in diameter from 3 to 0.8 mm after the continuous administration of topical vancomycin and ceftriaxone for 2 wk. Due to the slow healing, AMT was performed. Her corneal erosion healed and gradually became clear. Her visual acuity recovered from initially counting fingers to 100/200 at the last follow-up, 67 mo after AMT.
Antibiotics plus AMT may be an effective alternative treatment other than PK to promote epithelialization and to reduce inflammation in the corneas complicated by S. mitis keratitis.
Core Tip: In this case, we described the clinical and treatment course of an impending perforated corneal ulcer caused by Streptococcus mitis (S. mitis). We also demonstrated that treatment with antibiotics and amniotic membrane transplantation was successful, without the need for penetrating keratoplasty, and this could be considered an alternative treatment for nonhealing descemetoceles induced by S. mitis, as compared to the previous treatment.
- Citation: Hsiao FC, Meir YJJ, Yeh LK, Tan HY, Hsiao CH, Ma DHK, Wu WC, Chen HC. Amniotic membrane transplantation in a patient with impending perforated corneal ulcer caused by Streptococcus mitis: A case report and review of literature. World J Clin Cases 2022; 10(12): 3923-3929
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3923.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3923
Streptococcus mitis (S. mitis) is an alpha-hemolytic, facultative anaerobic species of the viridans group streptococci. S. mitis is a commensal of the human oropharynx and is also found on the skin, in the gastrointestinal tract, and in the female genital tract. Although the low virulence and pathogenicity of this bacteria are recognized, S. mitis is considered an opportunistic pathogen that can lead to the development of severe infections, including endophthalmitis, infective endocarditis, bacteremia, upper respiratory tract infection, and meningitis[1,2]. Moreover, corneal ulcers caused by S. mitis are rare and have seldom been described. In previous reports, penetrating keratoplasty (PK) was usually adopted for the treatment of persistent corneal ulcers[3-5].
As an alternative treatment to reconstruct the ocular surface, amniotic membrane transplantation (AMT) has been proposed to promote epithelial healing and to reduce neovascularization, inflammation, and scarring, and this method has been demonstrated to be effective in promoting wound healing and in preventing corneal perforation in infectious keratitis[6-9]. In this case, we demonstrated that AMT may be successfully used to treat a patient with a nonhealing descemetocele caused by S. mitis rather than performing PK.
A 63-year-old Taiwanese Han woman presented with right eye pain for 9 mo.
The patient had experienced right persistent corneal ulcers for 9 mo despite the use of biweekly therapeutic soft contact lenses along with unknown topical agents, which resulted in recurrent symptoms of ocular redness, pain, and blurred vision. Within a few years prior to the current event, she reported repeated episodes that occurred approximately two to three times yearly of right eye redness accompanied by photophobia that resolved spontaneously.
This patient had a history of herpes zoster ophthalmicus 18 years ago and an underlying disease of hypertension.
The patient denied any known family history.
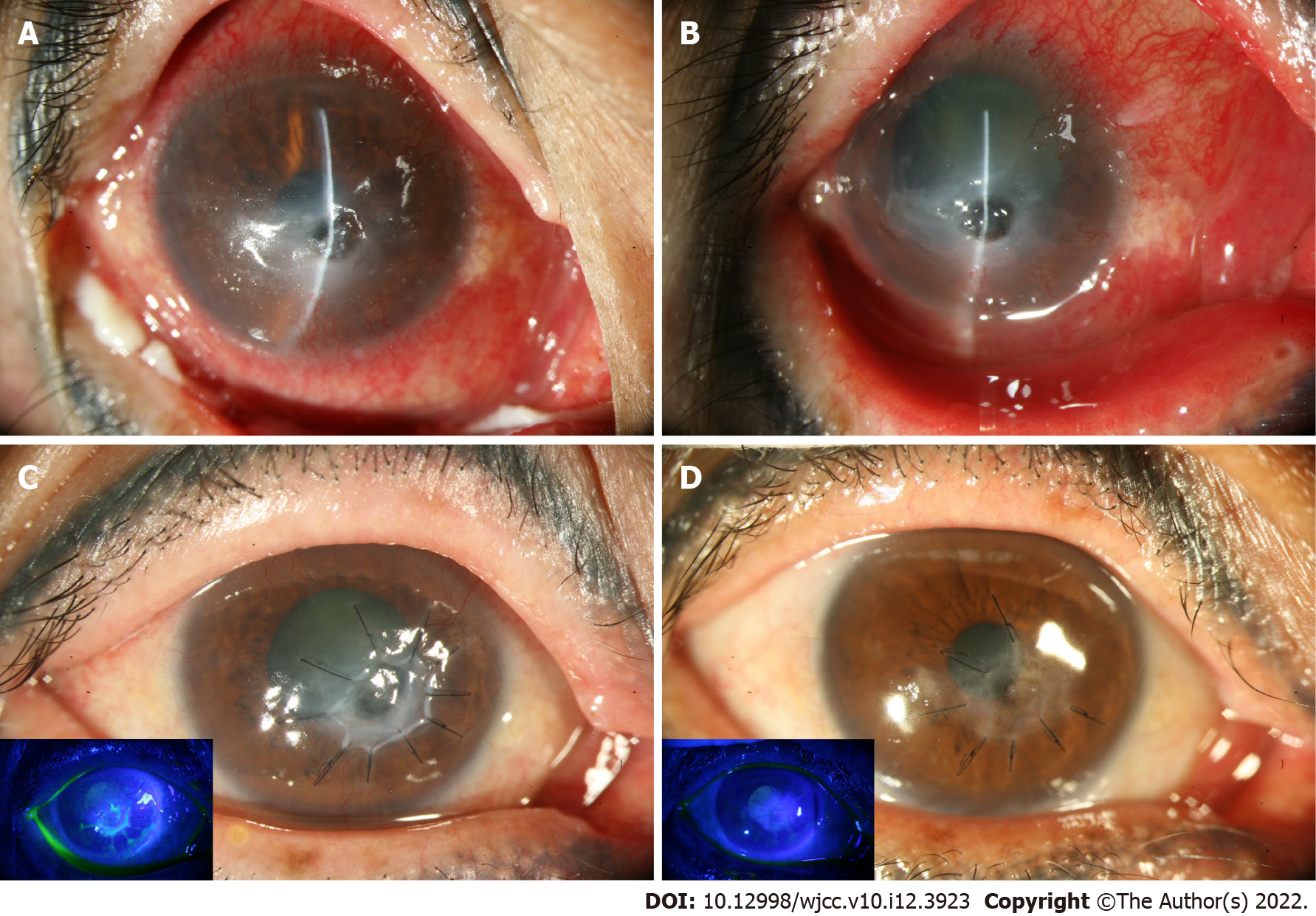
Upon the initial ocular examination, her right visual acuity (VA) was counting fingers. A 3 mm × 2 mm central epithelial defect with stromal infiltration and a 1 mm × 1 mm inferonasal paracentral descemetocele were noted at her right eye (Figure 1A). The VA change is listed in Table 1.
| Weeks after AMT | OD | OS | |
| −2 | VA | CF/15 cm | 0.5 |
| 1.6 | VA | CF/10 cm | 0.4–2 |
| 3.6 | VA | HM/60 cm | 0.3 |
| 6.6 | VA | CF/80 cm | 0.3 |
| 12.6 | VA | CF/30 cm | 0.5 |
| 20.6 | VA | CF/20 cm | 0.5 |
| 29.6 | VA | CF/10 cm | 0.7–2 |
| 37.6 | VAcPG | 0.05 | 1 |
| 54.6 | VAcPG | CF/30 cm | 1 |
| 63.6 | VAcPG | CF/20 cm | 0.8 |
| 73.6 | VA | 0.08 | 0.3 |
| 268.0 | VA | 100/200 | 0.5 |
A corneal culture yielded S. mitis growth.
Not applicable.
An impending perforated corneal ulcer was caused by S. mitis.
Famciclovir (250 mg, two tablets, TID), topical tobramycin ointment (3.5 g/tube, BID), and levofloxacin (0.5%, 25 mg/5 mL/bottle, Q1H) were prescribed initially. A subsequent corneal culture yielded S. mitis growth. Therefore, hourly topical vancomycin (25 mg/mL) and ceftriaxone (25 mg/mL) were initiated in place of the previous antiviral and antimicrobials based on the susceptibility test. AMT was performed after 2 wk of topical vancomycin and ceftriaxone.
The size of the descemetocele initially increased to 3 mm in diameter and was accompanied by the development of a 1 mm hypopyon. With the continuous administration of topical vancomycin and ceftriaxone for 2 wk, the descemetocele gradually decreased to 0.8 mm × 0.8 mm, and the hypopyon resolved (Figure 1B). Superficial manual keratectomy with AMT was performed[9] due to the minimal healing and the lack of further shrinkage of the descemetocele despite intensive topical antibiotic treatment (Figure 1C).
During the course of the corneal ulcer treatment, the patient reported an abrupt onset of left eye redness with abundant discharge. Pterygium at eight o’clock of the cornea and 360° chemosis with conjunctival injection (OS) were found. Topical sulfamethoxazole (4%, TID) and fluorometholone (0.1%, QID) were used, but the symptoms persisted. Therefore, the diagnostic aspiration of aqueous (OS) was performed. Fortunately, neither viral DNA nor organism was identified, and the severity of the chemosis and conjunctival injection gradually improved afterwards.
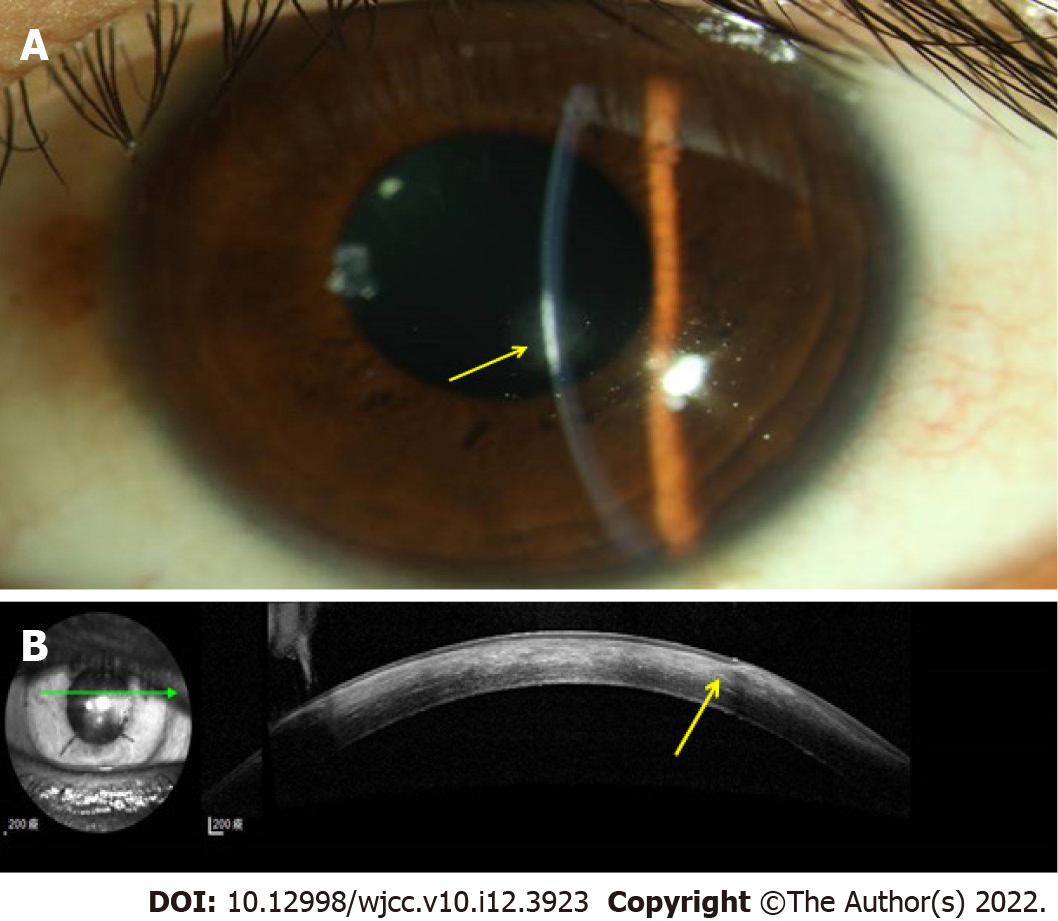
In a postoperative clinic follow-up, the amniotic membrane remained in situ without further epithelial defects or leakage at 6 mo post-AMT (Figure 1D). We switched the topical antibiotics to 0.5% levofloxacin and gradually tapered the dose. The cornea gradually healed with faint subepithelial haze as demonstrated by slit lamp biomicroscopy (Figure 2A) and anterior segment optical coherence tomography (Figure 2B), and best corrected VA was 100/200 at the last follow-up, 67 mo after the AMT was performed.
Well-documented treatments of S. mitis keratitis are rare, and most of the reported cases had poor visual outcomes or were treated with PK that were reported several years ago[3,5,10]. S. mitis is a normal flora of the human oropharynx and is also found on the skin, in the gastrointestinal tract, and in the female genital tract. Despite having low virulence and pathogenicity, reports have shown that S. mitis can cause severe infections, including endophthalmitis, infective endocarditis, bacteremia, upper respiratory tract infection, and meningitis[1,2]. This organism has been identified in patients with postsurgical endophthalmitis that resulted in poor visual outcomes[11]. In addition, the viridans group streptococci is one of the most common organisms implicated in the rare corneal infectious disease infectious crystalline keratopathy[12]. Although corneal ulcers caused by S. mitis have seldom been described, we treated the impending perforated ulcer with antibiotics for 2 wk before performing AMT.
Previously, in a 10-year review of microbial keratitis from 1972 to 1981, S. mitis was reported in 7% (3/44) of polymicrobial keratitis cases and in less than 5% of the 133 cases of monomicrobial keratitis[3]. The vision of one patient was limited to 2/200 by corneal scarring after antibacterial and antifungal therapies. The final vision of another patient was 10/200[3]. In 2005, there was a case report of a 39-year-old woman who presented with an S. mitis corneal ulcer with total corneal opacification and a 2.5 mm × 2.5 mm descemetocele. Antibiotics were used, but eventually, it progressed to a perforated cornea and was successfully treated with PK with a final VA of 20/200[4]. In 2016, another case was published of an S. mitis/oralis corneal ulcer that occurred 1 year after corneal transplantation. Although broad-spectrum antibiotics were given and infection was controlled, the corneal graft was complicated by scar formation. Regrafting was subsequently performed, and the new graft remained clear[5].
Giving initial topical empiric broad-spectrum antibiotics before available culture data is the general treatment of suppurative keratitis. Surgical treatment options include tissue adhesives, tarsorrhaphy, conjunctival flaps, and PK[13]. The management of a perforated corneal ulcer or descemetocele involves the repair of the mechanical disruption and the promotion of reepithelization while reducing inflammation[13]. AMT is an alternative treatment for reconstructing the ocular surface, and it has been proposed to be antimicrobial, to promote epithelial healing, and to reduce neovascularization, inflammation, and scarring[6,7].
AM is the innermost layer of the placenta composed of epithelium, basement membrane, and stroma. It was first used in ocular surface reconstruction in 1940 by de Rötth[14]. Later, few ophthalmologists adopted AM for surgery until Batlle and Perdomo used it for conjunctival reconstruction in the 1990s[15]. AMT can provide a physical barrier against infection and retention of antibiotics[16] and has antimicrobial properties via human beta-defensins[17], elafin[18], leukocyte protease inhibitor[19], and cystatin E[20]. In a rabbit model, AM was proven to subside pseudomonas keratitis[21]. A previous report also showed the effectiveness of AM in the treatment of infectious corneal ulcer[22].
As for mechanism of epithelial healing, the basement membrane of AM is similar to the conjunctiva that is mainly composed of type IV, V, and VII collagen that help the adhesion, migration, growth, and differentiation of epithelial progenitor cell[23,24]. The AM stroma contains several growth factors such as epidermal growth factor, hepatocyte growth factor, and basic fibroblast growth factor supporting epithelization[25-27]. In addition, protease inhibitors and heavy chain-hyaluronan/pentraxin 3 decrease the local inflammation and scarring[28,29]. In a recent meta-analysis, adjuvant AMT for infectious keratitis showed the promotion of corneal healing and the improvement of uncorrected VA[30].
The documented treatment for corneal ulcer caused by S. mitis was PK. While PK can resolve the pathology, it has the disadvantage of limited source of grafts and potential complications such as astigmatism, epithelial defects, and graft failure[8,9]. Considering the mechanism and effectiveness of AMT in infectious keratitis though not in S. mitis, we adopted AMT to treat the patient’s chronic infectious corneal ulcer. The patient’s final VA was good.
Comparing to documented treatment, PK for corneal ulcer caused by S. mitis, several studies have reported that AMT is effective in promoting corneal ulcer healing and in preventing corneal perforation in infectious keratitis though not in S. mitis. We presented a case of corneal ulcer caused by S. mitis treated by topical antibiotics with adjuvant AMT.
In this case, we described the clinical and treatment course of an impending perforated corneal ulcer caused by S. mitis. We also demonstrated that treatment with antibiotics and AMT was successful, without the need for PK, and this could be considered an alternative treatment for nonhealing descemetoceles induced by S. mitis, as compared to the previous treatment[3-5]. Given the current single case report, larger-scale studies are needed for AMT to become a standard treatment modality for persistent corneal ulcers prior to PK.
The authors thank the advice from Dr. Scheffer CG Tseng at the Ocular Surface Center, Miami, FL, United States.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dai Q, China; Khan I, Pakistan; Salvadori M, Italy S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Mitchell J. Streptococcus mitis: walking the line between commensalism and pathogenesis. Mol Oral Microbiol. 2011;26:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 140] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 2. | Chung JK, Lee SJ. Streptococcus mitis/oralis endophthalmitis management without phakic intraocular lens removal in patient with iris-fixated phakic intraocular lens implantation. BMC Ophthalmol. 2014;14:92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Jones DB. Polymicrobial keratitis. Trans Am Ophthalmol Soc. 1981;79:153-167. [PubMed] [Cited in This Article: ] |
| 4. | Hsu VJ, Affeldt J, Blanton C. Streptococcus Mitis Corneal Ulcer. Invest Ophthalmol Vis Sci. 2005;46:2632. [Cited in This Article: ] |
| 5. | Khan ID, Sati A, Arif S, Mehdi I, Bhatt P, Jain V, Konar J, Sahu C, Kumar Ramphal S, Pandit P. Streptococcus Mitis/Oralis Corneal Ulcer After Corneal Transplantation. J Basic Clin Med 2016; 5: 8-10. [Cited in This Article: ] |
| 6. | Hick S, Demers PE, Brunette I, La C, Mabon M, Duchesne B. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24:369-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 7. | Kim JS, Kim JC, Hahn TW, Park WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001;20:720-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 8. | Chen JH, Ma DH, Tsai RJ. Amniotic membrane transplantation for pseudomonal keratitis with impending perforation. Chang Gung Med J. 2002;25:144-152. [PubMed] [Cited in This Article: ] |
| 9. | Chen HC, Tan HY, Hsiao CH, Huang SC, Lin KK, Ma DH. Amniotic membrane transplantation for persistent corneal ulcers and perforations in acute fungal keratitis. Cornea. 2006;25:564-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 10. | Cristina N, SIzabela S, Ivan Ozana I. Complicated corneal ulcer. Case report. Rom J Ophthalmol. 2017;61: 239-243. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 11. | Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 275] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 12. | Khater TT, Jones DB, Wilhelmus KR. Infectious crystalline keratopathy caused by gram-negative bacteria. Am J Ophthalmol. 1997;124:19-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 13. | Dakhil TAB, Stone DU, Gritz DC. Adjunctive Therapies for Bacterial Keratitis. Middle East Afr J Ophthalmol. 2017;24:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (1)] |
| 14. | de Rötth A. Plastic Repair of Conjunctival Defects with Fetal Membranes. JAMA Ophthalmol. 1940;23:522-525. [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 258] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 15. | Batlle J, Perdomo F. Placental membranes as a conjunctival substitute. Ophthalmology. 1993;100:107. [Cited in This Article: ] |
| 16. | Ramuta TŽ, Starčič Erjavec M, Kreft ME. Amniotic Membrane Preparation Crucially Affects Its Broad-Spectrum Activity Against Uropathogenic Bacteria. Front Microbiol. 2020;11:469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 17. | Mao Y, Hoffman T, Singh-Varma A, Duan-Arnold Y, Moorman M, Danilkovitch A, Kohn J. Antimicrobial Peptides Secreted From Human Cryopreserved Viable Amniotic Membrane Contribute to its Antibacterial Activity. Sci Rep. 2017;7:13722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 19. | King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28:161-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Ni J, Abrahamson M, Zhang M, Fernandez MA, Grubb A, Su J, Yu GL, Li Y, Parmelee D, Xing L, Coleman TA, Gentz S, Thotakura R, Nguyen N, Hesselberg M, Gentz R. Cystatin E is a novel human cysteine proteinase inhibitor with structural resemblance to family 2 cystatins. J Biol Chem. 1997;272:10853-10858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Dallal MMS, Nikkhahi F, Imeni SM, Molaei S, Hosseini SK, Kalafi Z, Yazdi SS, Mirzaei HMA. Amniotic Membrane Transplantation for Persistent Epithelial Defects and Ulceration due to Pseudomonas Keratitis in a Rabbit Model. J Ophthalmic Vis Res. 2021;16:552-557. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Yin HY, Cheng AMS, Tighe S, Kurochkin P, Nord J, Dhanireddy S, Swan R, Alpert S. Self-retained cryopreserved amniotic membrane for treating severe corneal ulcers: a comparative, retrospective control study. Sci Rep. 2020;10:17008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73-79. [PubMed] [Cited in This Article: ] |
| 24. | Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z, Ma JX. Suppression of Corneal Neovascularization by PEDF Release from Human Amniotic Membranes. Invest Ophthalmol Vis Sci. 2004: 45: 1758-1762. [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Mahbod M, Shahhoseini S, Khabazkhoob M, Beheshtnejad AH, Bakhshandeh H, Atyabi F, Hashemi H. Amniotic Membrane Extract Preparation: What is the Best Method? J Ophthalmic Vis Res. 2014;9:314-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 26. | Mamede AC, Botelho MF. Amniotic membrane: origin, characterization and medical applications: Springer; 2015: IX, 254. [DOI] [Cited in This Article: ] |
| 27. | Stachon T, Bischoff M, Seitz B, Huber M, Zawada M, Langenbucher A, Szentmáry N. [Growth Factors and Interleukins in Amniotic Membrane Tissue Homogenate]. Klin Monbl Augenheilkd. 2015;232:858-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | He H, Li W, Tseng DY, Zhang S, Chen SY, Day AJ, Tseng SC. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009;284:20136-20146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Shay E, He H, Sakurai S, Tseng SC. Inhibition of angiogenesis by HC·HA, a complex of hyaluronan and the heavy chain of inter-α-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52:2669-2678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Ting DSJ, Henein C, Said DG, Dua HS. Amniotic membrane transplantation for infectious keratitis: a systematic review and meta-analysis. Sci Rep. 2021;11:13007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |