Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3647
Peer-review started: May 20, 2021
First decision: July 16, 2021
Revised: August 8, 2021
Accepted: March 4, 2022
Article in press: March 4, 2022
Published online: April 26, 2022
A growing body of evidence suggests that tight junction (TJ) proteins play a crucial role in the pathogenesis of various diseases, including gastrointestinal (GI) cancer and hepatocellular carcinoma (HCC). TJ proteins primarily maintain the epithelial and endothelial cells intact together through integral proteins however, recent reports suggest that they also regulate gene expression necessary for cell proliferation, angiogenesis, and metastasis through adapter proteins such as zonula occludens (ZO). ZO proteins are membrane-associated cytosolic scaffolding proteins that modulate cell proliferation by interacting with several transcription factors. Reduced ZO proteins in GI cancer and HCC are correlated with tumor development and poor prognosis. Pubmed has searched for using the keyword ZO and gastric cancer, ZO and cancer, and ZO and HCC for the last ten years to date. This review summarized the role of ZO proteins in cell proliferation and their expression in GI cancer and HCC. Furthermore, therapeutic interventions targeting ZO in GI and liver cancers are reviewed.
Core Tip: Zonula occludens (ZO) proteins (ZO-1, -2, -3) are primarily involved in tight junction formation. Additionally, ZO proteins regulate cell proliferation by interacting with various transcription factors, among which ZO/ZO-1 associated nucleic acid-binding protein pathway is of particular importance. Reduced expression of ZO proteins is correlated with poor prognosis in gastrointestinal cancer and hepatocellular carcinoma. Modulating ZO proteins expression with polyphenols, probiotics and peptides may represent promising therapeutic agents for cancer treatment.
- Citation: Ram AK, Vairappan B. Role of zonula occludens in gastrointestinal and liver cancers. World J Clin Cases 2022; 10(12): 3647-3661
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3647.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3647
Tight junction (TJ) proteins are intercellular cell adhesion molecules located at the apical region of epithelial and endothelial cells and firmly seal the gap between adjacent cells. TJ proteins confer cell polarity and prevent the free flow of lipids and proteins within the cell membrane preventing their translocation from apical to the basolateral domain. It also forms a mechanical barrier to restrict the movement of solutes, ions, and water through the paracellular pathway, which is essential for physiological homeostasis in tissues and organs[1]. Recent data suggest that apart from gate and fence function, TJ proteins have also been linked to communicate various signaling cascades through adapter proteins and regulate gene expression necessary for cell proliferation and differentiation[2]. TJ is composed of a dynamic set of protein complexes which includes integral transmembrane proteins such as claudins, occludin, and cytosolic scaffolding proteins such as zonula occludens (ZO) proteins ZO-1, ZO-2, and ZO-3.
ZO proteins interconnect actin-cytoskeleton with transmembrane TJ proteins and contribute to cellular adhesion[3]. Moreover, in the cells deprived of TJ, ZO proteins were seen to be attached with cadherin-dependent adherens junction through alpha-catenin. In addition, ZO proteins also interact with gap junctions by directly binding with connexin (Cx) proteins such as Cx-36, Cx-43 and Cx-45[4]. The ZO proteins belong to members of a subfamily of membrane-associated guanylate kinase (MAGUK) proteins, share some common structural domains such as PSD/disc large/ZO-1 (PDZ) domain, Src Homology 3 (SH3) domain, and catalytically inactive guanylate kinase (GUK) domain. These domains interact with the homologous domain of other proteins and form a mutual protein-protein interaction for various cellular processes. Importantly, the PDZ domain is required to polymerize claudins and occludin at the junctional site to stabilize TJ[5]. These domains also interact with various cytoplasmic proteins, transcription factors, and signaling molecules to regulate gene expression[6]. These functions of ZO proteins pinpoint a critical role not only in the maintenance of intercellular adhesion and communication but also suggest a non-canonical function in various cellular growth and differentiation. Perturbation or disruption of TJ proteins expression is associated with the progression of a variety of cancers, including gastrointestinal (GI) cancer such as stomach cancer, colon cancer, and hepatocellular carcinoma (HCC)[7].
Furthermore, various cytokines, growth factors, pathogens, and miRNA are known to regulate TJ proteins expression[2]. In addition, TJ proteins also maintain the intestinal epithelial barrier integrity and any compromise in intestinal permeability due to inflammation or gut bacterial overgrowth contributing initiation or progression of GI cancer[8]. Here, we mainly focused on the role of ZO proteins in GI and liver cancers progression and possible therapeutic intervention for the clinical outcome.
ZO-1 is the first identified 220 kD peripheral cytoplasmic protein consisting of three PDZ domains, an SH3 domain, catalytically inactive GUK domain, and alternatively spliced a carboxy half terminal which binds to actin filament[3]. ZO-2 and ZO-3 (160 kD and 130 kD, respectively) proteins contain a similar domains and bind to ZO-1 via their corresponding PDZ-2 domain[3]. Besides these domains, ZO proteins also contain variable motifs U1 to U6 inter-located between PDZ, SH3, and GUK domains. PDZ domains are evolutionarily conserved regions found in bacteria, fungi, mammals, and plant lineages consisting of 80-100 amino acid residues[6]. These domains form dimers with corresponding PDZ domains, attached to C-terminal sequences of integral membrane proteins or cytoplasmic proteins and play a key role in epithelial cellular integrity and signal transduction. This PDZ containing proteins is specifically localized at the intercellular junction of epithelial cells, the cell membrane of lymphocyte and erythrocyte and neuromuscular junctions, and maintains cellular adhesion, regulation of paracellular transport, and intracellular signaling[6].
The first PDZ domain of all the ZO proteins binds to the C-terminal sequence of claudins. In contrast, the second and third PDZ domains interact with junction adhesion molecule (JAM), which is critical for constructing TJ and its function[1]. Indeed, Umeda et al[5] showed polymerization of claudins at TJ is functionally regulated by ZO-1 and ZO-2 PDZ binding domains. Similarly, the SH3 domain consists of a short segment of around 60 amino acid residues which was first found in viral adapter protein c-Crk as the conserved sequence. This domain was later found in several cytosolic tyrosine kinases and non-catalytic parts of phospholipase enzymes. The SH3 domain participates in protein-protein interaction by recognizing proline-rich ligands of cytoskeletal proteins, Src kinases, and various other proteins regulating a wide array of biological functions such as enzyme activity, formation of multimeric protein complexes, and assembly of the cytoskeleton[9]. The SH3-U5-GUK-U6 domain of ZO-1 binds to occludin and stabilizes its polymerization at TJ. The U5 motif present at the hinge region is imperative for ZO-1-occludin stabilization and localization of ZO-1 at TJ[10]. The GUK domain in the presence of ATP converts GMP to GDP. Moreover, other MAGUK family proteins such as p55 and Lin-2, where this domain is catalytically active and contains GMP and ATP binding sites, the ZO-1 GUK domain lacks this function. It is believed that the catalytic activity of the GUK domain was gradually lost with the evolutionary emergence of subfamily members of MAGUK proteins, as demonstrated by phylogenetic analysis[4]. Previous studies showed that the GUK domain also participates in inter-protein interaction and several proteins such as brain enriched guanylate kinase-associated protein (BEGAIN), microtubule-associated protein (MAP1A), and kinesin-like protein (GAKIN) containing GUK binding ligands. Furthermore, the GUK domain also communicates intramolecularly with the SH3 domain[10].
Moreover, when the cells lacking the U6 motif encompassing the GUK domain of ZO-1 were introduced, the ectopic TJ strands consisting of claudin and occludin were formed. However, it failed to recruit cytoplasmic junctional plaque molecules[10]. In addition, ZO proteins serve as an essential intermediate molecule linking TJ strands with actomyosin cytoskeleton, where actin filament interacts with ZO proteins through their carboxy-terminal (ZO-1 and ZO-2) and N-terminal end (ZO-3)[11].
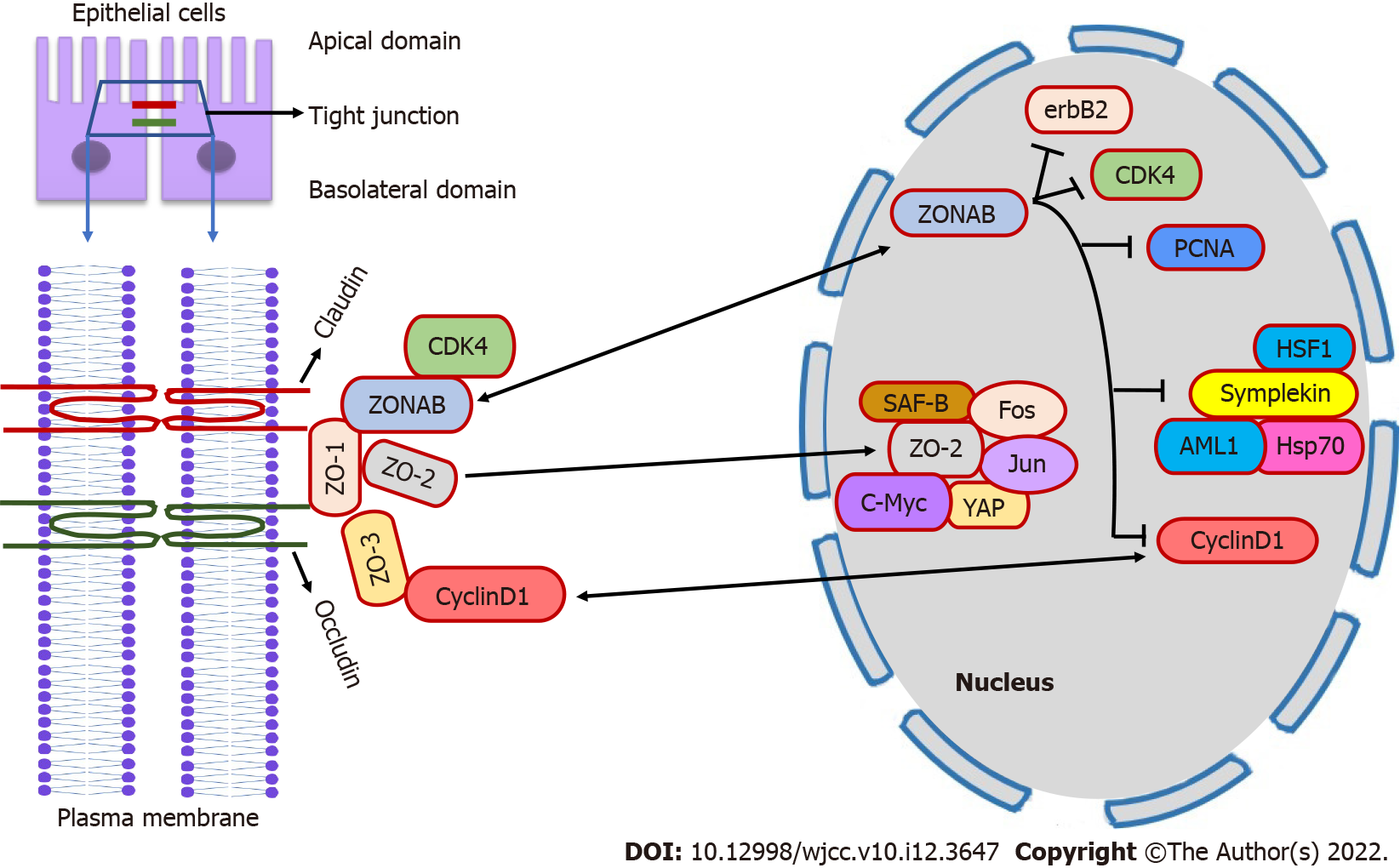
ZO proteins are confined to maintaining TJ integrity and govern cell proliferation, maturation, and cell cycle transition. The drosophila’s Disc large (Dlg) protein, a member of MAGUK family, shares homology with mammalian ZO-1 in which the SH3 domain has tumor suppressor activity. Therefore, ZO-1 might play a role as a tumor suppressor protein. Consequently, beta catenin-mediated reduced ZO-1 expression is associated with enhanced proliferation of colorectal epithelial cells[12]. In contrast, the ectopic introduction of ZO-1 showed reduced cell proliferation and transformation in MDCK cells[13]. Moreover, ZO-1 knockout mice showed embryonic lethality due to abnormal angiogenesis and embryonic cells apoptosis in the yolk sac[13]. Apart from protein-binding domains, ZO-1 contains nuclear localization signal (NLS) and nuclear export signal (NES). Thus, it can scaffold between plasma membrane and nucleus, suggesting direct involvement in gene transcription[10]. Gottardi et al[14] showed nuclear expression of ZO-1 before TJ maturation in proliferating subconfluent cell culture of epithelial cells. However, not all investigators have found the nuclear expression of ZO-1, and its role in the nucleus remains elusive. One of the mechanisms by which ZO-1 regulates cell proliferation might be the binding of ZO-1 with transcription factor ZO-1 associated nucleic acid-binding protein (ZONAB)[15].
ZONAB is a multifunctional Y-box transcription factor containing sequences of inverted CAAT box that bind to the SH3 domain of ZO-1 protein and regulate gene expression and cell proliferation[16]. A previous study in MDCK cells shows that cell density determines the cytosolic and nuclear fraction of ZO-1 and ZONAB[15]. Thus, in high proliferating low-density cells, the ZONAB is highly expressed in the nucleus while; ZO-1 expression is reduced at the junctional site[15]. Ectopic overexpression of ZO-1 or by ZONAB RNA interference reduces the nuclear pool of ZONAB, thereby inhibiting MDCK cell proliferation[15]. Moreover, enhanced expression of ZONAB through lentivirus or knockdown of ZO-1 by RNA interference increased the proliferation of retinal pigment epithelial (RPE) cells associated with epithelial to mesenchymal transition (EMT) of RPE cells[17]. The above study suggests that ZO-1 might inhibit cell proliferation by cytoplasmic concealment of ZONAB at the junctional site and proves that nuclear accumulation of ZONAB depends on cellular proliferation rate. However, a study by Spadaro et al[18] confirmed that ZO-1 alone is not responsible for ZONAB activity and cellular content; rather, it also requires ZO-2 and ZO-3. Therefore, reduction of any one of ZO proteins has a barren effect on subcellular localization of ZONAB, whereas reduced expression of both ZO-1 and ZO-2 is coupled with concomitant reduction of ZO-3 preventing junctional localization of ZONAB and reinforcing its transition to the nucleus[18].
ZO-1/ZONAB axis regulates cell proliferation by interacting with proteins involved in cell cycle progressions such as cyclin-dependent kinase 4 (CDK4), cyclin D1 (CD1), and proliferating cell nuclear antigen (PCNA)[15,19]. ZONAB promotes nuclear accumulation of CDK4, thus causing the transition of the cell cycle progression from G1 to S phase while ZO-1 sequestering ZONAB at the junctional site prevents the accumulation of CDK4 in the nucleus[15]. ZONAB also induces transcription of CD1 and PCNA genes through the CCAAT inverted promoter sequence resulting in epithelial cell proliferation while the ZO-1-SH3 domain reduces the cellular protein content of CD1[18]. ZO-1 also regulates erbB2 protooncogene transcription[15]. ZONAB act as a repressor of erbB2 protooncogene, which is involved in cellular growth and neoplastic transformation and its expression is highly increased in cancer cells[15]. However, in non-transformed cells, overexpression of ZO-1 reduces cell proliferation by alleviating endogenous erbB2 levels[15]. The erbB2 gene is involved in cell differentiation and organogenesis[20]. Symplekin, an mRNA polyadenylation factor, is another target of ZONAB through which it regulates gene expression and inhibits cell differentiation[21]. Nuclear symplekin accumulation interacts with heat shock inducible transcription factor 1 (HSF1) and induces polyadenylation of heat-shock protein 70 (Hsp70) mRNA[22]. In the colon epithelial cells, ZONAB-symplekin complex also represses the activity transcription factor acute myeloid leukemia 1 (AML1) protein inhibiting cell differentiation[10]. Apg-2 is a stress-responsive molecule that interacts with the ZO-1 through the SH3 domain and regulates ZONAB transcriptional functions as both Apg-2 and ZONAB competitively bind to the same SH3 domain of ZO-1. Accordingly, in MDCK cells, heat shock treatment or overexpression of Apg-2 activates transcriptional activity of ZONAB and stimulates cell proliferation, while Apg-2 silencing favors colocalization of ZONAB with ZO-1at the junctional site[10].
ZO-2 also shares structural features common to ZO-1, in addition, it also possesses NLS and NES[23]. An embryonic lethality with altered yolk sac angiogenesis and apoptosis was also observed in ZO-2 knockout mice similar to ZO-1[24]. However, when ZO-2(-/-) chimeric mice were generated, these mice were viable with altered blood-testis barrier without affecting the levels of other TJ proteins such as claudin, occludin, ZO-1, and ZO-3. These results suggest that ZO-2 is not required for proper embryogenesis rather, it is vital for the development of extraembryonic tissues and the formation of the blood-testis barrier[25]. ZO-2 is the only ZO subfamily protein transiently expressed in the nucleus. ZO-2 accumulates inside the nucleus in sparse culture whereas, in confluent monolayer cells, ZO-2 is seen at TJ strands in MDCK and endothelial cells[23]. Additionally, ZO-2 regulates cell proliferation by binding various transcription factors such as c-myc, fos, Jun, and C/EBP, and SAF-B[26]. In particular, ZO-2 inhibits cell cycle progression by inhibiting c-myc dependent RNA transcription and protein degradation of CD1, thus arresting the cell cycle at G0/G1 phase[27]. Furthermore, the PDZ domains of ZO-2 binds to Armadillo repeated gene deleted in velo-cardio-facial syndrome (ARVCF) protein which is closely related to the p120 catenin family proteins and colocalize at TJ in non-proliferating cells while breach of intercellular adhesion leads to nuclear accumulation of ARVCF where it induces gene expression by activating kaiso protein[10,28].
In addition, at the epithelial junction, the carboxy-terminal of ZO-2 binds to hScrib protein which is homologous to tumor suppressor scribble protein in drosophila, suggesting its tumor suppressor role[29]. ZO-2 also interacts with another transcription factor Yes-associated protein (YAP) of the Hippo signaling pathway through the PDZ domains and regulates the nuclear accumulation of YAP for gene expression[30]. Accordingly, the knockdown of ZO-2 promoted renal hypertrophy by activating the nuclear accumulation of YAP[31]. Formation of TJ strand at cell junction required dual expression of ZO-1 and ZO-2. Umeda et al[5] showed that inhibition of ZO-1 and ZO-2 expression by RNA interference leads to altered claudin polymerization with impaired TJ strand formation. In contrast, the ectopic introduction of ZO-1 and ZO-2 corrected conformational claudin polymerization and restored TJ formation in ephrin-4 (EpH4) cells. Similarly, when coexpression of ZO-1 and ZO-2 were depleted in the embryo, the TJ establishment was altered in extraembryonic endothelial cells, which were crucial for normal cavitation and survival of the embryo, whereas individual deletion did not affect TJ integrity[32]. In addition, increased paracellular permeability was observed in MDCK cells by dual depletion ZO-1 and ZO-2[4].
TJ ZO-3 binds to ZO-1 via the PDZ2 domain and not to ZO-2. Unlike other ZO proteins (ZO-1 and ZO-2), the mice lacking ZO-3 have no embryonic lethality, and TJ strands were completely formed, suggesting ZO-3 is nonobligatory for TJ assembly[24]. However, Kiener et al[33] showed that ZO-3 is imperative for normal TJ construction in the epidermis layer of zebrafish embryos. Furthermore, aberrant expression of ZO-3 has been proclaimed in the number of cancers such as breast cancer, suggesting its additional role in cell proliferation, however, the mechanism has yet to be identified[34]. One possible mechanism may be the knockdown of ZO-3 resulted in protein degradation of CD1 and arrested cell cycle at G0/G1 phase inhibiting cell proliferation[13]. As discussed above, ZO proteins are critical for TJ assembly and control cell proliferation by interacting with various transcription factors and cell cycle-related genes (Figure 1).
Tissue microarray results showed enhanced expression of ZO-1 staining in gastric intestinal-type adenocarcinoma, whilst reduced ZO-1 expression in diffused gastric adenocarcinoma[35,36]. Moreover, compared to normal gastric mucosa, reduced ZO-1 immunostaining was observed in gastric cancer patients[37,38]. Similarly, high cytoplasmic expression of ZO-1 was reported in GI stromal tumor (GIST) tissue indicating the malignant phenotype of GIST and might serve as a favorable prognosis in GIST patients[39]. In addition, ZO-1 and ZO-2 were also depleted in scirrhous gastric carcinoma cells (OCUM-12) derived from patients with scirrhous gastric carcinoma[40]. Furthermore, ZONAB expression was upregulated in gastric cancer tissue while silencing of ZONAB in gastric cancer cell line decreased expression of adenomatous polyposis coli (APC), CD1, and E-cadherin and inhibited cell proliferation[41].
The shreds of evidence from previous studies in gastric cancer cells have shown that various factors regulated the expression of ZO proteins. For example, Helicobacter pylori Cag protein redistributed intercellular ZO-1 to small vesicles in primary gastric epithelial cells[42]. Similarly, in TMK-1 cells, a poorly differentiated gastric carcinoma cell line, ZO-1 staining was observed in cytoplasm while the addition of fresh serum or epidermal growth factor (EGF) relocated the ZO-1 from cytosol to the cell membrane, which was mediated by protein kinase C (PKC)[43]. Similarly, in noncancerous gastric epithelial cell line IMGE-5, hepatocyte growth factor (HGF) treatment causes translocation of ZO-1 from the TJ strand to cytosol and nucleus, thereby increasing transepithelial resistance, accompanied by phosphatidylinositol 3-kinase (PI3K) dependent ZO-1 tyrosine phosphorylation. This phosphorylation prevented the binding of ZO-1 to occludin, suggesting that HGF hinders the accumulation of ZO-1 at TJ amid cell differentiation[44]. In another study, HGF treatment to gastric epithelial cell line MKN74, the cytoplasmic translocation of ZO-1 was observed, which was associated with cell migration rather than increased cell proliferation. In addition, they also found increased tyrosine phosphorylation of occludin, whereas ZO-1 phosphorylation was not affected, and paracellular permeability was not compromised. However, ZO-1/occludin interaction was affected[45]. Moreover, circular RNAs (circRNAs) circSMC3 promoted gastric cancer cells proliferation and motility by targeting ZO-1[46].
In primary colorectal cancer (CRC) tissue loss of ZO-1 expression was found, whereas liver metastasized tissues showed reexpression of ZO-1. The decreased expression of ZO-1 in CRC was due to tyrosine phosphorylation of ZO-1 mediated by epidermal growth factor receptor (EGFR), while ZO-1 in liver metastasized tissue was found to be dephosphorylated. This indicates that phosphorylation of ZO-1 reduces its functional capacity, which is necessary for glandular dedifferentiation in CRC acquiring invasive properties of CRC tissue, whereas glandular redifferentiation liver metastasis might require reexpression of ZO-1[47]. Similarly, in colon cancer biopsy tissue, decreased expression of ZO-1 was positively correlated with high tumor grade and poor outcome in CRC patients[48]. Furthermore, in colon adenocarcinoma tissue, reduced ZO-1expression was correlated with tumor cell differentiation[49]. In patients with colitis-associated colorectal carcinoma (CAC), ZO-1 expression was not altered compared to adjoining intraepithelial neoplastic tissue and normal mucosa[50]. Liu et al[51] showed increased expression of ZONAB in CRC, which was associated with cell invasion, degree of differentiation, and metastasis in those patients. Conversely, knockdown of ZONAB by short-hairpin RNA impeded cell proliferation, enhanced apoptotic activity, halted cell cycle progression in CRC cell lines, and inhibited tumor growth in xenograft mice. In addition, knockdown of ZONAB in CRC cells upregulated the expression of p38, suggesting the involvement of ZONAB in CRC progression through MAPK pathways, however, further investigation is needed to correlate ZONAB expression and CRC development[52].
Past studies from in vitro models suggested that ZO proteins were regulated by various factors in the pathogenesis of colon cancer. ZO-1 expression was decreased in various colon cancer cell lines[12]. ZO-1 was expressed at the apical border of T84 colon cancer cells and in the cytoplasm of COLO320DM cells, while in DLD-1 cells, ZO-1 was expressed at the cell border showing an intermediate epitheloid phenomenon between T84 cells and DLD-1. These observations indicated that the ZO-1 expression is associated with epitheloid disorganization in colon cancer[53]. Upregulated miR-103 promoted CRC by targeting ZO-1 and binds to 3’ UTR of ZO-1[54]. Similarly, miR-200b upregulated the ZO-1 expression in Caco-2 cells via myosin light chain kinase phosphorylation and alleviated tumor necrosis factor-alpha (TNF-α) induced interleukin-8 (IL-8) secretion[55]. Moreover, overexpression of miR-212 decreased ZO-1 expression in Caco-2 cells, while lentivirus-mediated knockdown of miR-212 increased ZO-1 expression[56]. Furthermore, upregulated miR-191a, decreased protein and mRNA expression of ZO-1 in intestinal epithelial cells (IEC-6), whereas its inhibition increased TNF-α induced cell injury[57]. Cancer secreted exosomal miR-25-3-p promoted CRC metastasis to liver and lung in mice by downregulating ZO-1, kruppel like factor (KLF) 2, and KLF4 expression and by upregulating vascular endothelial growth factor receptor 2 expression[58]. In addition, long non-coding RNAs, AFAP1-AS1 knockdown inhibited colon cancer cell lines proliferation by upregulating ZO-1[59]. Similarly, ZC3H13, a CCCH zinc finger protein, inhibited the proliferation of CRC cells by upregulating ZO-1. The reduced expression of ZC3H13 in CRC specimens was associated with TNM stage and lymph node metastasis[60]. Deoxycholic acid downregulated ZO-1 expression via NLRP3 inflammasome in Caco-2 cells and promoted colon carcinogenesis[61]. Patulin, a mycotoxin present in the food, phosphorylate and lowers the ZO-1 expression in the colon cancer Caco-2 cell line, promoting colon cancer[62]. Acetylsalicylic acid (NSAIDs) decreased ZO-1 expression through reactive oxygen mediated in Caco-2 cells[63].
ZO proteins are a critical component of TJ and are involved in maintaining intestinal barrier integrity. Recent studies demonstrated increased intestinal permeability associated with colon cancer progression[64,65]. Inflammation is a precipitating factor for intestinal permeability. For example, inflammatory cytokine TNF-α treatment downregulated the expression of ZO-1 in colon cancer Caco-2 cells and induced intestinal permeability. This was mediated by activating the nuclear factor kappa B (NF-κB) transcription factor. Subsequently, inhibition of NF-κB activity modulated TNF-α expression and reappearance of ZO-1 at the apical border in Caco-2 cells[2]. Similarly, IL-15 enhanced the expression of ZO-1 and ZO-2 in the colon cancer T84 cell line and improved the TJ barrier[66]. Estrogen treatment reduced gene and protein expression of ZO-1, including its promoter activity in Caco-2 cells, mediated by NF-κB activation[67]. Similarly, intestinal alkaline phosphatase (IAP) increased the expression of ZO-1 and ZO-2 in colon cancer Caco-2 and T84 cell lines. In addition, IAP ameliorated lipopolysaccharide (LPS)-induced inflammation and relocation of ZO-1 at TJ improving epithelial barrier integrity[68]. Furthermore, Phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3) inhibited the expression of ZO-1 in Caco-2 cells and decreased TJ barrier permeability which was improved after PIK3R3 inhibitor TAT-N 15[69]. Transcription factor JunD repressed ZO-1 expression through cAMP response element-binding protein-binding (CREB) in colon cancer Caco-2 cells impairing intestinal barrier integrity[30]. The above studies suggested that inflammation is a prime mediator of alteration in TJ protein expression contributing to intestinal permeability.
In the liver, ZO proteins are localized in the hepatocytes and hepatic endothelial cells, forming the blood biliary barrier. It is also highly expressed in the cholangiocytes forming the intact intrahepatic bile duct. Altered expression of ZO proteins or mislocalization of ZO proteins plays a crucial role in liver biology. The hepatic knockout of ZO-1 and ZO-2 in rodents did not show major organ abnormalities at birth but was lethal after 6 wk. However, distinct abnormal features in the liver were observed in those knockout mice where hepatocyte polarity was lost with a concomitant reduction in bile duct structure and disorganized sinusoidal vessels indicating ZO-1 and ZO-2 are necessary for normal liver physiological function[70]. Accordingly, Takaki et al[71] showed that after 2/3rd partial hepatectomy, expression of ZO-1 was reduced, and it reappeared after 3 d indicating ZO-1 is critical for liver regeneration. Orbán et al[72] reported reduced ZO-1 mRNA expression in HCC liver tissue and colorectal liver metastasis tissue compared to the normal liver sample. Furthermore, ZO-1 cellular localization was observed in the HCC liver showing reduced positivity, whereas colorectal metastasis liver tissue showed increased positivity attributing to glandular dedifferentiation. Another study by Zhang et al[73] showed reduced mRNA and protein expression of ZO-1 in HCC tissue compared to adjacent non-tumorous tissue. Accordingly, overexpression of ZO-1 inhibited HepG2 cell proliferation and blocked the cell cycle transition at the G1 interphase.
Furthermore, reduced expression of ZO-1 was associated with shorter overall survival in patients with HCC after partial hepatectomy[74]. In our previous study in HCC patients, decreased hepatic ZO-1 expression was correlated with poor outcomes. A positive interrelationship was observed between increased plasma ZO-1 concentration and hsCRP, indicating inflammation disrupts TJ protein expression in HCC patients[75]. Similarly, in the diethylnitrosamine (DEN) and N-nitrosomorpholine (NMOR) induced HCC mouse model, we found reduced hepatic ZO-1 protein expression. In contrast, ZONAB expression was increased, indicating ZO-1/ZONAB pathway plays a critical role in HCC pathogenesis[76]. Similarly, Ponziani et al[77] showed increased plasma ZO-1 levels correlated with inflammatory markers in HCC patients compared to healthy volunteers. ZO-2 and ZO-3 expressions data are scarce in liver cancer, indeed, reduced expression of ZO-2 was associated with progressive familial intrahepatic cholestasis (PFIC) type 4[78,79]. Similarly, a missense mutation was identified in the first PDZ domain of ZO-2 in patients with familial hypercholanemia[80]. In addition, expression of ZONAB was increased in HCC tissue which was correlated with advanced stages of HCC. In contrast, the nuclear ZONAB expression in HCC tissue showed a poor prognosis in HCC patients. Moreover, this nuclear localization was caused by T to G transversion and methylation of the ZONAB promoter region[81,82]. Hypoxia-inducible factor-1αmediated upregulation of miR-191 promoted ischemia/reperfusion liver injured through ZONAB/CyclinD1 axis[83]. In this context, Gao et al[84] showed Insulin-like growth factor II mRNA-binding protein 3 (IGF2BP3) enhanced HCC cell invasion by upregulating miR-195-5p induced suppression of ZO-1 expression. Table 1 summarizes the expression of ZO proteins in gastric cancer, colon cancer, and HCC under clinical settings.
| Ref. | ZO-protein | Specimen and methods | Observation | Potential clinical relevance |
| Resnick et al[35] | ZO-1 | Tissue sample by microarray staining | Increased expression in gastric intestinal-type adenocarcinoma while Reduced expression in diffuse gastric cancer | Associated with tumor differentiation |
| Kimura et al[36] | ZO-1 | Tissue sample by immunohistochemistry | Reduced expression in poorly differentiated gastric adenocarcinoma | Correlated with tumor differentiation |
| Lee et al[37] | ZO-1 | Tissue sample by immunohistochemistry | Reduced expression in diffuse gastric cancer | Associated with tumor differentiation |
| Ohtani et al[38] | ZO-1 | Tissue sample by immunohistochemistry | Reduced expression in undifferentiated-type gastric adenocarcinoma | Associated with reduced overall survival |
| Zhu et al[39] | ZO-1 | Tissue sample by microarray staining | Increased expression in gastrointestinal stromal tumor | Positively correlated with longer survival |
| Wang et al[41] | ZONAB | Tissue sample by western blotting and immunofluorescence | Increased expression in gastric cancer tissue | Knockdown of ZONAB by siRNA inhibited cyclin D1 mediated cell invasion and enhanced chemosensitivity of 5-fluorouracil |
| ZO-2 and ZO-3 | Not studied in gastric cancer patients | |||
| Kaihara et al[47] | ZO-1 | Tissue sample by immunohistochemistry | Reduced expression in primary colorectal cancer | Reduced expression is attributed by tyrosine phosphorylation of ZO-1 and is required for dedifferentiation of glandular structure in CRC |
| Resnick et al[48] | ZO-1 | Tissue sample by microarray staining | Reduced expression in colon cancer | Low expression is correlated with high tumor grade |
| Jeong et al[49] | ZO-1 | Tissue sample by immunohistochemistry | Reduced expression in colon adenocarcinoma | Reduced expression is correlated with tumor cell differentiation |
| Mees et al[50] | ZO-1 | Tissue sample by immunofluorescence | No difference in expression between colorectal carcinoma, adjoining intraepithelial neoplasia, and normal mucosa | Unknown |
| Orbán et al[72] | ZO-1 | Tissue sample by PCR and immunohistochemistry | Reduced mRNA and protein expression in primary HCC; Increased protein expression in secondary colorectal liver metastasis tissue | Differential expression of ZO-1 is associated with distinct histological features in these tumors |
| Liu et al[51] | ZONAB | Tissue sample by immunohistochemistry | Increased expression in CRC | Increased expression is correlated with invasion, degree of differentiation, and colorectal metastasis |
| ZO-2 and ZO-3 | Not studied in colorectal cancer patients | |||
| Zhang et al[73] | ZO-1 | Tissue sample by PCR and western blotting | Reduced mRNA and protein expression in HCC | Overexpression of ZO-1 inhibited HepG2 cell proliferation |
| Nagai et al[74] | ZO-1 | Tissue sample by PCR | Reduced mRNA expression in HCC | Reduced expression is correlated with shorter overall survival and poor prognosis in HCC patients |
| Ram et al[75] | ZO-1 | Tissue sample by immunohistochemistry; Plasma sample by ELISA | Reduced expression in HCC; Increased plasma concentration in HCC | Associated with inflammation and poor clinical outcome in HCC patients |
| Ram et al[76] | ZO-1 | Tissue sample by western blotting | Reduced expression in HCC | Associated with inflammation and disease pathogenesis in DEN and NMOR induced HCC |
| Yasen et al[81] | ZONAB | Tissue sample by immunohistochemistry | Increased expression in HCC liver tissue | Nuclear staining of ZONAB is correlated with poor prognosis in HCC patients |
| ZO-2 and ZO-3 | Not studied in HCC patients |
As discussed above, ZO proteins play multiple roles in cancer progression, including cell proliferation, metastasis, and invasion, thereby contributing to the pathogenesis of GI and liver cancers. Targeting TJ proteins such as ZO may ablate disease progression.
Other than ZO proteins that were downregulated, the transmembrane TJ proteins such as claudin family proteins were upregulated in a variety of GI cancer. Accordingly, in recent years several antibodies targeting claudins proteins have been developed. Monoclonal antibodies (mAB) against claudin 1, 2, 3, 4, 6 and 18.2. has been generated and is under the preclinical and clinical stages[7]. For example, mAB 6F6 against claudin 1 is in the preclinical stage for CRC treatment, whereas the claudiximab, mAB against claudin 18.2 is in a clinical trial for gastric cancer therapy[7]. Since several claudins proteins were found to be upregulated in GI cancer, mAB therapy might be useful, however, ZO proteins are cytosolic scaffolding proteins and were found to be downregulated in GI cancer. The same approach may be difficult to apply for the treatment of GI cancers. Therefore, the compounds which can directly target ZO proteins and regulate their expression might represent a promising candidate for ZO targeted therapy. One such compound 4,-hydroxyphenylmethylene hydantoin (PMH), a non-toxic compound isolated from marine sponge, was able to attenuate prostate cancer growth and prevent distance metastasis by preventing TJ disruption, accompanied by upregulation of ZO-1 expression[85]. Further studies are needed to identify small molecules targeting ZO proteins that can directly modulate their expression, which might be useful for the treatment of GI cancer.
Most of the current generation of anticancer drugs are targeted to the cell surface receptor or intracellular kinases, modulation of cytosolic protein-protein interactions mediated by non-enzymatic domains is an underexplored area for the development of new anti-cancer chemotherapeutic agents. One such domain is a PDZ domain that possesses non-enzymatic actions through which several proteins interact and transmit signal transduction for cellular homeostasis. ZO proteins contain evolutionarily conserved PDZ domains. Hence, targeting the PDZ domain to modulate protein-protein interaction with small molecule inhibitors or peptides might block the cellular signaling pathways required for cancer growth. Accordingly, various small molecule inhibitors or peptides regulating PDZ domains have been identified[86]. For example, ZL006 and IC87201, a small molecule inhibitor of PDZ domain of PSD-95 (postsynaptic density) protein, prevented binding of neuronal nitric oxide synthase (nNOS) and attenuated cerebral ischemia brain injury when administered in mice[86]. Similarly, a mammalian protein named GIPC (GAIP-interacting protein, C terminus) contains a central PDZ domain that regulates insulin-like growth factor-1 receptor (IGF-1R) expression through PDZ-domain dependent[87]. Octapeptide CR1023 (N-myristoyl-PSQSSSEA), a GIPC-PDZ inhibitor, attenuated the pancreatic cancer growth with a significant reduction in IGF-1R expression. CR1166 [N-myristoyl-PSQSK(εN-4-bromobenzoyl) SK (εN-4-bromobenzoyl)A] another peptide inhibitor of GIPC-PDZ, inhibited the cell proliferation and induced apoptosis in pancreatic and breast cancer cells as well as in the xenograft model. CR1166 prevented the interaction between GIPC-PDZ domain and IGF-1R with a concomitant reduction in protein expression of IGF-1R and epidermal growth factor receptor (EGFR)[88]. Further, small molecule inhibitors like NSC668036, J01-017a, and FJ9 have been identified targeting PDZ domains of Disheveled (Dvl) proteins, an important regulator of wnt/β catenin pathway[86]. These compounds blocked the interaction of the Dvl-PDZ domain with frizzled proteins, hampering the downstream wnt/β catenin signaling pathway, which propagate the carcinogenesis process[86]. However, these compounds have not been tested for the selective inhibition of PDZ domains of ZO proteins. It will be interesting to see whether these compounds can modulate ZO-PDZ protein-protein interaction binding partners. Further studies are needed to develop new small molecules or peptide modulators to examine the specific regulation of the PDZ domain of ZO proteins which can be implemented for cancer therapy.
Reduced expression of ZO-1 is associated with intestinal permeability, which contributes to colon cancer and HCC progression[89]. Therefore, compounds that can modify the expression of ZO proteins and preserve barrier integrity might be employed to prevent CRC and HCC progression. Previous studies have shown that various polyphenols can modulate ZO-1 protein expression and preserve TJ barrier integrity in vitro and in vivo[90]. In HT-29 colon epithelial cells, treatment with red polyphenol upregulated ZO-1 expression and attenuated cytokine-stimulated intestinal barrier permeability[91]. Resveratrol treatment enhanced the expression of ZO-1 and preserved TJ barrier integrity both in vivo and in vitro[92]. Curcumin treatment restored intestinal epithelial barrier integrity by upregulating ZO-1 expression in colon epithelial cells[93]. In addition, curcumin also attenuated TNF-α induced intestinal ischemia/reperfusion injury by increasing the ZO-1 expression in Caco-2 cells[94]. Kaempferol, a flavonoid improved intestinal TJ barrier integrity by upregulating ZO-1 expression in colon cancer cell lines[95]. Berberine is a natural antioxidant that increases ZO-1 expression in colon epithelial tissue and reduces LPS mediated TJ barrier permeability[96]. Proanthocyanidin, a grape seed extract, ameliorated LPS-induced inflammation and oxidative stress by improving barrier integrity by upregulating ZO-1 expression in Caco-2 cells[97]. Treatment with curcumin, quercetin, and naringenin reduced dextran sodium sulphate-induced colitis in mice and also reduced colonic barrier permeability by upregulating ZO-1 protein expression[98]. In our previous study, nimbolide, a terpenoid, increased hepatic ZO-1 protein expression in HCC mice and ameliorated disease pathogenesis. Moreover, we also found ZO-1 has a ligand-binding cavity for nimbolide[76]. Furthermore, we also found nimbolide treatment restores intestinal barrier integrity by upregulating ZO-1 expression in HCC mice [unpublished data]. Vitamin D3 supplementation upregulated the expression of ZO-1 via the beta-catetin-TCF-4 pathway in colon cancer cells[99]. Similarly, treatment with retinoic acid in colitis mice with compromised gut barrier significantly upregulated ZO-1 expression and improved TJ permeability[100]. Lycopene a carotenoid inhibited the cell proliferation of human cutaneous squamous cell carcinoma cell line COLO-16 by upregulating ZO-1 expression[101]. However, limited studies have been conducted in gastric cancer, colon cancer, and in HCC animal models directly targeting ZO proteins with natural compounds, which might inhibit the carcinogenesis process, which remains an unexplored area.
Previous studies have provided substantial evidence that prebiotic can modulate TJ proteins and might preserve intestinal barrier integrity for the prevention of disease pathogenesis[102]. Administration of inulin fermentation products preserved tissue barrier integrity by upregulating ZO-1 mRNA expression in intestinal epithelial cells[103]. Similarly, treatment with fructo-oligosachharide and butyrate upregulated the ZO-1 expression and enhanced barrier integrity in Caco-2 cells[104,105]. Furthermore, supplementation of galacto-oligosaccharide attenuated inflammatory response and intestinal permeability by upregulating ZO-1 gene expression in LPS-challenged mice[106]. In vivo study showed that dietary tryptophan supplementation improved intestinal barrier permeability by upregulating ZO-1 expression[107].
Probiotics represent another approach to restore TJ barrier integrity. Accordingly, probiotic E. coli Nissle1971 supplementation to DSS-induced colitis mice markedly improved barrier permeability by upregulating ZO-1 expression[108]. Furthermore, treatment with probiotic VSL#3 upregulated ZO-1 expression and enhanced barrier integrity in various cancer models both invitro in vivo[109,110]. VSL#3 treatment to DEN-induced HCC rats restored mucosal barrier integrity and alleviated tumor burden[111]. Lactobacillus plantarum ZLP001 and Lactobacillus reuteri I5007 alleviated inflammation and strengthened intestinal barrier integrity by upregulating ZO-1 expression in vitro and in vivo[112,113]. Enterococcus faecium HDRsEf1 treatment protected LPS-induced intestinal epithelial cell injury model by upregulating ZO-1 expression in IPEC-J2 cells[114]. However, the use of prebiotics and probiotics supplementation in rodent models of gastric cancer, CRC, and HCC specifically targeting ZO proteins is limited, and further investigation is required.
Histone deacetylase (HDAC) enzymes regulate gene expression by histone modification and altering the chromatin structure. In addition, HDAC participates in post-translational modification of proteins by acetylation and deacetylation, and thus enhances or represses the activity of proteins. It was reported that HDAC inhibitors modulate TJ protein expression[115]. In support of this, treatment with HDAC inhibitor sodium butyrate to rat fibroblast cells significantly increased ZO-1 and ZO-2 expression by inhibiting HDAC activity and favoring cell differentiation. However, further studies are needed to delineate the mechanism of HDAC inhibitor-mediated upregulation of ZO proteins to treat GI cancer and HCC[116].
In conclusion, ZO proteins are shown to critical for preserving barrier integrity by communicating with various transcription factors, thereby regulating cell proliferation, differentiation, and cell cycle progression. Moreover, ZO proteins were distinguished as tumor suppressor genes. Importantly, the ZO-1/ZONAB pathway was found to be crucial in the TJ-mediated regulation of gene expression. In addition, reduced expression of ZO-1 was correlated with poor prognosis in patients with gastric, colon and liver cancers. However, scarce literature shows the role of ZO-2 and ZO-3 in GI cancer. Therefore, modulation of ZO proteins expression by small molecules or peptides and gene transfer may represent a potential candidate for cancer treatment. Additionally, natural polyphenols, prebiotics and probiotics also show potential therapeutic intervention for the modulation of ZO-1 expression in GI cancer and HCC pathogenesis. Indeed, future studies are warranted to delineate the mechanism of alteration of ZO proteins expression in GI cancer and HCC and subsequent development to the new chemotherapeutic drug.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver, No. 11313.
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qiu F, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Piontek J, Krug SM, Protze J, Krause G, Fromm M. Molecular architecture and assembly of the tight junction backbone. Biochim Biophys Acta Biomembr. 2020;1862:183279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Bhat AA, Uppada S, Achkar IW, Hashem S, Yadav SK, Shanmugakonar M, Al-Naemi HA, Haris M, Uddin S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front Physiol. 2018;9:1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 3. | Otani T, Furuse M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020;30:805-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 4. | Heinemann U, Schuetz A. Structural Features of Tight-Junction Proteins. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 5. | Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 592] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 6. | Lee HJ, Zheng JJ. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. 2010;8:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 7. | Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut. 2019;68:547-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 696] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 9. | Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114:1253-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 605] [Cited by in F6Publishing: 605] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179-35185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 349] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/Lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 600] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 13. | Gonzalez-Mariscal L, Miranda J, Ortega-Olvera JM, Gallego-Gutierrez H, Raya-Sandino A, Vargas-Sierra O. Zonula Occludens Proteins in Cancer. Curr Pathobiol Rep. 2016;4:107-116. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci U S A. 1996;93:10779-10784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024-2033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K, Balda MS, Ali RR. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One. 2010;5:e15730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S. ZO proteins redundantly regulate the transcription factor DbpA/ZONAB. J Biol Chem. 2014;289:22500-22511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387-2398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Troyer KL, Lee DC. Regulation of mouse mammary gland development and tumorigenesis by the ERBB signaling network. J Mammary Gland Biol Neoplasia. 2001;6:7-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Kavanagh E, Buchert M, Tsapara A, Choquet A, Balda MS, Hollande F, Matter K. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci. 2006;119:5098-5105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Xing H, Mayhew CN, Cullen KE, Park-Sarge OK, Sarge KD. HSF1 modulation of Hsp70 mRNA polyadenylation via interaction with symplekin. J Biol Chem. 2004;279:10551-10555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Islas S, Vega J, Ponce L, González-Mariscal L. Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp Cell Res. 2002;274:138-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669-1678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Xu J, Anuar F, Ali SM, Ng MY, Phua DC, Hunziker W. Zona occludens-2 is critical for blood-testis barrier integrity and male fertility. Mol Biol Cell. 2009;20:4268-4277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, González-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res. 2004;292:51-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Tapia R, Huerta M, Islas S, Avila-Flores A, Lopez-Bayghen E, Weiske J, Huber O, González-Mariscal L. Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle. Mol Biol Cell. 2009;20:1102-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614-3623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 326] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Métais JY, Navarro C, Santoni MJ, Audebert S, Borg JP. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett. 2005;579:3725-3730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Oka T, Remue E, Meerschaert K, Vanloo B, Boucherie C, Gfeller D, Bader GD, Sidhu SS, Vandekerckhove J, Gettemans J, Sudol M. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Domínguez-Calderón A, Ávila-Flores A, Ponce A, López-Bayghen E, Calderón-Salinas JV, Luis Reyes J, Chávez-Munguía B, Segovia J, Angulo C, Ramírez L, Gallego-Gutiérrez H, Alarcón L, Martín-Tapia D, Bautista-García P, González-Mariscal L. ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Mol Biol Cell. 2016;27:1581-1595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Phua DC, Xu J, Ali SM, Boey A, Gounko NV, Hunziker W. ZO-1 and ZO-2 are required for extra-embryonic endoderm integrity, primitive ectoderm survival and normal cavitation in embryoid bodies derived from mouse embryonic stem cells. PLoS One. 2014;9:e99532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Kiener TK, Selptsova-Friedrich I, Hunziker W. Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos. Dev Biol. 2008;316:36-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717-2725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Resnick MB, Gavilanez M, Newton E, Konkin T, Bhattacharya B, Britt DE, Sabo E, Moss SF. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol. 2005;36:886-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, Monden M. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151:45-54. [PubMed] [Cited in This Article: ] |
| 37. | Lee SK, Moon J, Park SW, Song SY, Chung JB, Kang JK. Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol Rep. 2005;13:193-199. [PubMed] [Cited in This Article: ] |
| 38. | Ohtani S, Terashima M, Satoh J, Soeta N, Saze Z, Kashimura S, Ohsuka F, Hoshino Y, Kogure M, Gotoh M. Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastric Cancer. 2009;12:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Zhu H, Lu J, Wang X, Zhang H, Tang X, Zhu J, Mao Y. Upregulated ZO-1 correlates with favorable survival of gastrointestinal stromal tumor. Med Oncol. 2013;30:631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Kato Y, Yashiro M, Noda S, Tendo M, Kashiwagi S, Doi Y, Nishii T, Matsuoka J, Fuyuhiro Y, Shinto O, Sawada T, Ohira M, Hirakawa K. Establishment and characterization of a new hypoxia-resistant cancer cell line, OCUM-12/Hypo, derived from a scirrhous gastric carcinoma. Br J Cancer. 2010;102:898-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Wang GR, Zheng Y, Che XM, Wang XY, Zhao JH, Wu KJ, Zeng J, Pan CE, He DL. Upregulation of human DNA binding protein A (dbpA) in gastric cancer cells. Acta Pharmacol Sin. 2009;30:1436-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Krueger S, Hundertmark T, Kuester D, Kalinski T, Peitz U, Roessner A. Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathol Res Pract. 2007;203:433-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Yoshida K, Kanaoka S, Takai T, Uezato T, Miura N, Kajimura M, Hishida A. EGF rapidly translocates tight junction proteins from the cytoplasm to the cell-cell contact via protein kinase C activation in TMK-1 gastric cancer cells. Exp Cell Res. 2005;309:397-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Hollande F, Blanc EM, Bali JP, Whitehead RH, Pelegrin A, Baldwin GS, Choquet A. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol. 2001;280:G910-G921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Nasu Y, Ido A, Tanoue S, Hashimoto S, Sasaki F, Kanmura S, Setoyama H, Numata M, Funakawa K, Moriuchi A, Fujita H, Sakiyama T, Uto H, Oketani M, Tsubouchi H. Hepatocyte growth factor stimulates the migration of gastric epithelial cells by altering the subcellular localization of the tight junction protein ZO-1. J Gastroenterol. 2013;48:193-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Xia T, Pan Z, Zhang J. CircSMC3 regulates gastric cancer tumorigenesis by targeting miR-4720-3p/TJP1 axis. Cancer Med. 2020;9:4299-4309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Kaihara T, Kawamata H, Imura J, Fujii S, Kitajima K, Omotehara F, Maeda N, Nakamura T, Fujimori T. Redifferentiation and ZO-1 reexpression in liver-metastasized colorectal cancer: possible association with epidermal growth factor receptor-induced tyrosine phosphorylation of ZO-1. Cancer Sci. 2003;94:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2005;18:511-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Jeong YK, Lee MJ, Lim SC, Kee KH, Jeon HJ, Suh CH. Expression of Osteopontin, ZO-1 and E-cadherin in Adenoma and Adenocarcinoma of the Colon. Korean J Pathol 2005; 39: 242-250. [Cited in This Article: ] |
| 50. | Mees ST, Mennigen R, Spieker T, Rijcken E, Senninger N, Haier J, Bruewer M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int J Colorectal Dis. 2009;24:361-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Liu RT, Wang GR, Liu C, Qiu J, Yan LK, Li XJ, Wang XQ. RNAi-mediated downregulation of DNA binding protein A inhibits tumorigenesis in colorectal cancer. Int J Mol Med. 2016;38:703-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Liu R, Wang G, Liu C, Qiu J, Yan L, Li X, Wang X. Gene expression profile analysis of dbpA knockdown in colorectal cancer cells. Cell Biol Int. 2016;40:1280-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. |
Sasaki K, Kokai Y, Atsumi S, Tobioka H, Sawada N, Hirata K, et al Difference in the expression of three tight junction proteins, barmotin, occludin, and ZO-1, in phenotypically different human colon cancer cell lines.
|
| 54. | Ke J, Shao W, Jiang Y, Xu J, Li F, Qin J. MicroRNA103 regulates tumorigenesis in colorectal cancer by targeting ZO1. Mol Med Rep. 2018;17:783-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Shen Y, Zhou M, Yan J, Gong Z, Xiao Y, Zhang C, Du P, Chen Y. miR-200b inhibits TNF-α-induced IL-8 secretion and tight junction disruption of intestinal epithelial cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2017;312:G123-G132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Tang Y, Zhang L, Forsyth CB, Shaikh M, Song S, Keshavarzian A. The Role of miR-212 and iNOS in Alcohol-Induced Intestinal Barrier Dysfunction and Steatohepatitis. Alcohol Clin Exp Res. 2015;39:1632-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Wang L, Zhang R, Chen J, Wu Q, Kuang Z. Baicalin Protects against TNF-α-Induced Injury by Down-Regulating miR-191a That Targets the Tight Junction Protein ZO-1 in IEC-6 Cells. Biol Pharm Bull. 2017;40:435-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J, Zhu X, Yang W, Liao W, Li G, Ding Y, Liang L. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 569] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 59. | Bo H, Fan L, Li J, Liu Z, Zhang S, Shi L, Guo C, Li X, Liao Q, Zhang W, Zhou M, Xiang B, Li G, Xiong W, Zeng Z, Xiong F, Gong Z. High Expression of lncRNA AFAP1-AS1 Promotes the Progression of Colon Cancer and Predicts Poor Prognosis. J Cancer. 2018;9:4677-4683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 60. | Zhu D, Zhou J, Zhao J, Jiang G, Zhang X, Zhang Y, Dong M. ZC3H13 suppresses colorectal cancer proliferation and invasion via inactivating Ras-ERK signaling. J Cell Physiol. 2019;234:8899-8907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 61. | Liu L, Dong W, Wang S, Zhang Y, Liu T, Xie R, Wang B, Cao H. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018;9:5588-5597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 62. | Kawauchiya T, Takumi R, Kudo Y, Takamori A, Sasagawa T, Takahashi K, Kikuchi H. Correlation between the destruction of tight junction by patulin treatment and increase of phosphorylation of ZO-1 in Caco-2 human colon cancer cells. Toxicol Lett. 2011;205:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Fukui A, Naito Y, Handa O, Kugai M, Tsuji T, Yoriki H, Qin Y, Adachi S, Higashimura Y, Mizushima K, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Yagi N, Kokura S, Yoshikawa T. Acetyl salicylic acid induces damage to intestinal epithelial cells by oxidation-related modifications of ZO-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G927-G936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 247] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Diao XY, Peng T, Kong FG, Huang JG, Han S, Shang YS, Liu H. Alcohol consumption promotes colorectal cancer by altering intestinal permeability. Eur Rev Med Pharmacol Sci. 2020;24:9370-9377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 66. | Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem. 2001;276:35571-35580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Zhou Z, Zhang L, Ding M, Luo Z, Yuan S, Bansal MB, Gilkeson G, Lang R, Jiang W. Estrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin Immunol. 2017;183:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Liu W, Hu D, Huo H, Zhang W, Adiliaghdam F, Morrison S, Ramirez JM, Gul SS, Hamarneh SR, Hodin RA. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J Am Coll Surg. 2016;222:1009-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Ibrahim S, Zhu X, Luo X, Feng Y, Wang J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int Immunopharmacol. 2020;85:106610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 70. | Itoh M, Terada M, Sugimoto H. The zonula occludens protein family regulates the hepatic barrier system in the murine liver. Biochim Biophys Acta Mol Basis Dis. 2021;1867:165994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Takaki Y, Hirai S, Manabe N, Izumi Y, Hirose T, Nakaya M, Suzuki A, Mizuno K, Akimoto K, Tsukita S, Shuin T, Ohno S. Dynamic changes in protein components of the tight junction during liver regeneration. Cell Tissue Res. 2001;305:399-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Orbán E, Szabó E, Lotz G, Kupcsulik P, Páska C, Schaff Z, Kiss A. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res. 2008;14:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Zhang X, Wang L, Zhang H, Tu F, Qiang Y, Nie C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol Lett. 2019;17:1859-1864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Nagai T, Arao T, Nishio K, Matsumoto K, Hagiwara S, Sakurai T, Minami Y, Ida H, Ueshima K, Nishida N, Sakai K, Saijo N, Kudo K, Kaneda H, Tamura D, Aomatsu K, Kimura H, Fujita Y, Haji S, Kudo M. Impact of Tight Junction Protein ZO-1 and TWIST Expression on Postoperative Survival of Patients with Hepatocellular Carcinoma. Dig Dis. 2016;34:702-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Ram AK, Pottakat B, Vairappan B. Increased systemic zonula occludens 1 associated with inflammation and independent biomarker in patients with hepatocellular carcinoma. BMC Cancer. 2018;18:572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Ram AK, Vairappan B, Srinivas BH. Nimbolide inhibits tumor growth by restoring hepatic tight junction protein expression and reduced inflammation in an experimental hepatocarcinogenesis. World J Gastroenterol. 2020;26:7131-7152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 374] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 78. | Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, Logan CV, Newbury LJ, Kamath BM, Ling S, Grammatikopoulos T, Wagner BE, Magee JC, Sokol RJ, Mieli-Vergani G; University of Washington Center for Mendelian Genomics, Smith JD, Johnson CA, McClean P, Simpson MA, Knisely AS, Bull LN, Thompson RJ. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 79. | Ge T, Zhang X, Xiao Y, Wang Y, Zhang T. Novel compound heterozygote mutations of TJP2 in a Chinese child with progressive cholestatic liver disease. BMC Med Genet. 2019;20:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, Morton DH, Bull LN. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Yasen M, Kajino K, Kano S, Tobita H, Yamamoto J, Uchiumi T, Kon S, Maeda M, Obulhasim G, Arii S, Hino O. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res. 2005;11:7354-7361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Yasen M, Obulhasim G, Kajino K, Mogushi K, Mizushima H, Tanaka S, Tanaka H, Hino O, Arii S. DNA binding protein A expression and methylation status in hepatocellular carcinoma and the adjacent tissue. Int J Oncol. 2012;40:789-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Pan W, Wang L, Zhang XF, Zhang H, Zhang J, Wang G, Xu P, Zhang Y, Hu P, Zhang XD, Du RL, Wang H. Hypoxia-induced microRNA-191 contributes to hepatic ischemia/reperfusion injury through the ZONAB/Cyclin D1 axis. Cell Death Differ. 2019;26:291-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Gao Y, Luo T, Ouyang X, Zhu C, Zhu J, Qin X. IGF2BP3 and miR191-5p synergistically increase HCC cell invasiveness by altering ZO-1 expression. Oncol Lett. 2020;20:1423-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Shah GV, Muralidharan A, Thomas S, Gokulgandhi M, Mudit M, Khanfar M, El Sayed K. Identification of a small molecule class to enhance cell-cell adhesion and attenuate prostate tumor growth and metastasis. Mol Cancer Ther. 2009;8:509-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Christensen NR, Čalyševa J, Fernandes EFA, Lüchow S, Clemmensen LS, Haugaard-Kedström LM, Strømgaard K. PDZ Domains as Drug Targets. Adv Ther (Weinh). 2019;2:1800143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 87. | Muders MH, Vohra PK, Dutta SK, Wang E, Ikeda Y, Wang L, Udugamasooriya DG, Memic A, Rupasinghe CN, Baretton GB, Aust DE, Langer S, Datta K, Simons M, Spaller MR, Mukhopadhyay D. Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth. Clin Cancer Res. 2009;15:4095-4103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Patra CR, Rupasinghe CN, Dutta SK, Bhattacharya S, Wang E, Spaller MR, Mukhopadhyay D. Chemically modified peptides targeting the PDZ domain of GIPC as a therapeutic approach for cancer. ACS Chem Biol. 2012;7:770-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 89. | Lee B, Moon KM, Kim CY. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J Immunol Res. 2018;2018:2645465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 90. | Ram AK, Wright G, Vairappan B. Microbiota, Inflammation, and Gut Barrier Dysfunction in HCC. In: Vijay Gayam and Omer Engin. Liver Pathology. Intechopen, 2019. [DOI] [Cited in This Article: ] |
| 91. | Nunes C, Freitas V, Almeida L, Laranjinha J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: implications for intestinal inflammation. Food Funct. 2019;10:1364-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 92. | Mayangsari Y, Suzuki T. Resveratrol Ameliorates Intestinal Barrier Defects and Inflammation in Colitic Mice and Intestinal Cells. J Agric Food Chem. 2018;66:12666-12674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 93. | Wang J, Ghosh SS, Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am J Physiol Cell Physiol. 2017;312:C438-C445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 94. | Tian S, Guo R, Wei S, Kong Y, Wei X, Wang W, Shi X, Jiang H. Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-α related mechanism. Korean J Physiol Pharmacol. 2016;20:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Suzuki T, Tanabe S, Hara H. Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. J Nutr. 2011;141:87-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 96. | Gu L, Li N, Li Q, Zhang Q, Wang C, Zhu W, Li J. The effect of berberine in vitro on tight junctions in human Caco-2 intestinal epithelial cells. Fitoterapia. 2009;80:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Nallathambi R, Poulev A, Zuk JB, Raskin I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 98. | Shigeshiro M, Tanabe S, Suzuki T. Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis. J Funct Foods. 2013;5:949-955. [Cited in This Article: ] |
| 99. | Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 591] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 100. | Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. Cellular retinoic acid bioavailability determines epithelial integrity: Role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol. 2007;71:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Bi S, Li L, Gu H, Li M, Xu S, Bu W, Zhang M, Zhou Z, Chen X. Lycopene upregulates ZO-1 and downregulates claudin-1 through autophagy inhibition in the human cutaneous squamous cell carcinoma cell line COLO-16. J Cancer. 2019;10:510-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 756] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 103. |
Uerlings J, Schroyen M, Willems E, Tanghe S, Bruggeman G, Bindelle J, et al Differential effects of inulin or its fermentation metabolites on gut barrier and immune function of porcine intestinal epithelial cells.
|
| 104. | Wongkrasant P, Pongkorpsakol P, Ariyadamrongkwan J, Meesomboon R, Satitsri S, Pichyangkura R, Barrett KE, Muanprasat C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed Pharmacother. 2020;129:110415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 105. | Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1032] [Cited by in F6Publishing: 1147] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 106. | Wang G, Sun W, Pei X, Jin Y, Wang H, Tao W, Xiao Z, Liu L, Wang M. Galactooligosaccharide pretreatment alleviates damage of the intestinal barrier and inflammatory responses in LPS-challenged mice. Food Funct. 2021;12:1569-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Liu W, Mi S, Ruan Z, Li J, Shu X, Yao K, Jiang M, Deng Z. Dietary Tryptophan Enhanced the Expression of Tight Junction Protein ZO-1 in Intestine. J Food Sci. 2017;82:562-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, Suerbaum S, Buer J, Gunzer F, Westendorf AM. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 319] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 109. | Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. 2012;29:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 110. | Cheng FS, Pan D, Chang B, Jiang M, Sang LX. Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases. World J Clin Cases. 2020;8:1361-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (4)] |
| 111. | Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q, Chen HY, Dai RY, Qiu BJ, He YQ, Wang C, Zheng LY, Li YQ, Wu FQ, Li Z, Yan HX, Wang HY. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 112. | Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, Wang Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front Microbiol. 2018;9:1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 113. | Yang F, Wang A, Zeng X, Hou C, Liu H, Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 114. | Liu L, Li Y, He Y, Wang Z, Zhao H, Jin X, Shi D, Wang X. Enterococcus faecium HDRsEf1 inhibits Lipopolysaccharide-induced downregulation of zona occludens -1 expression via toll-like receptor 2/4-mediated c-Jun N-terminal kinase/activator protein-1 signalling pathways. J Appl Microbiol. 2022;132:605-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 115. | Vinken M, Peggy P, Vera R, Tamara V. Histone deacetylase inhibitors as potent modulators of cellular contacts. Curr Drug Targets. 2006;7:773-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Bordin M, D'Atri F, Guillemot L, Citi S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol Cancer Res. 2004;2:692-701. [PubMed] [Cited in This Article: ] |