Published online Oct 26, 2018. doi: 10.12998/wjcc.v6.i12.548
Peer-review started: June 11, 2018
First decision: June 20, 2018
Revised: July 11, 2018
Accepted: August 11, 2018
Article in press: August 12, 2018
Published online: October 26, 2018
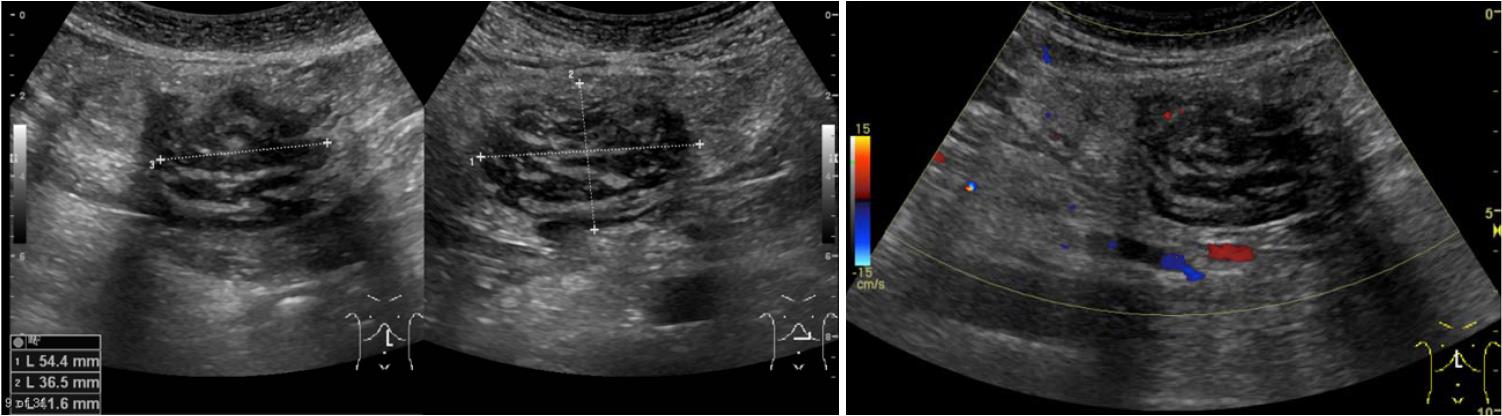
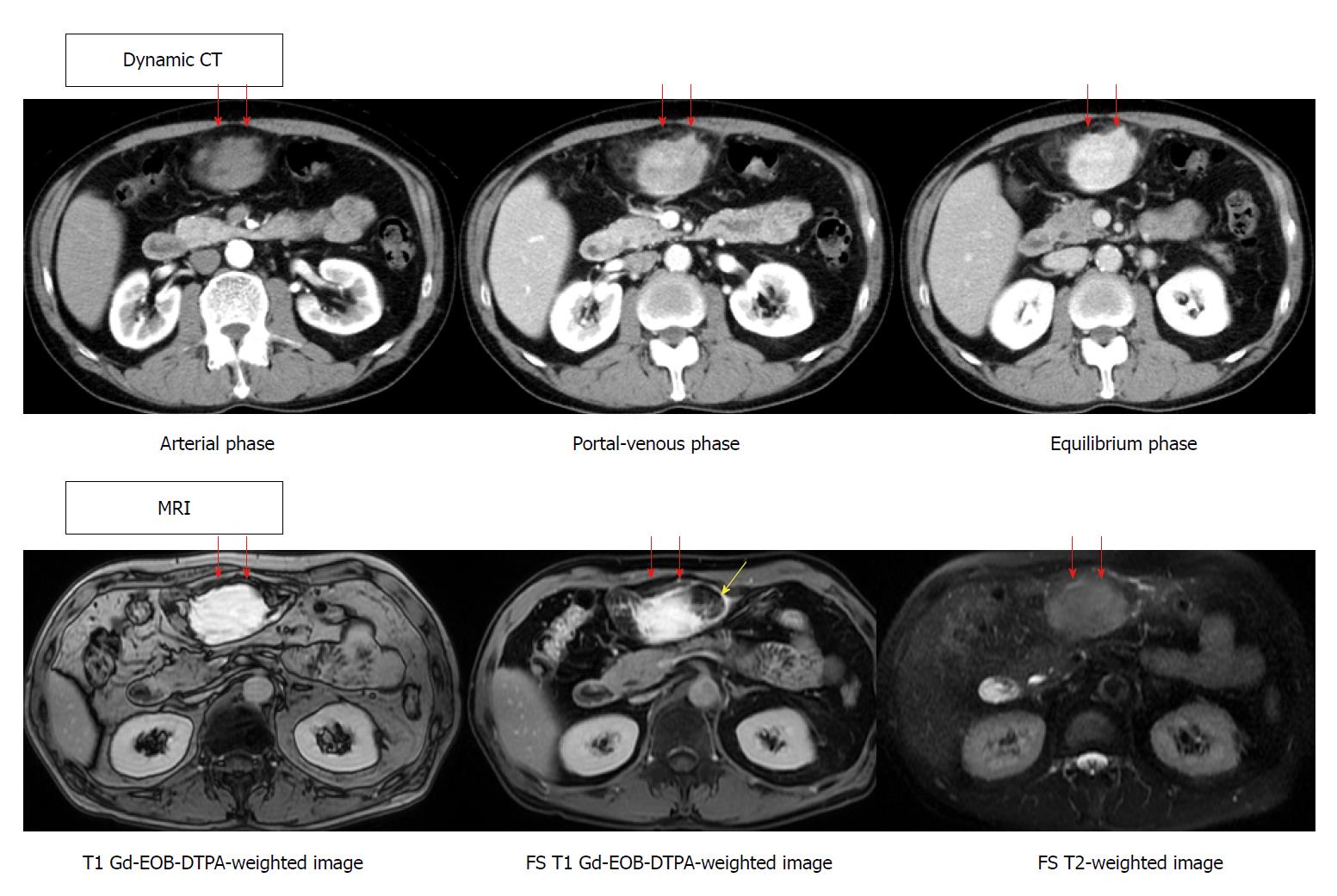
A 61-year-old male was referred to our hospital with a three-month history of persistent epigastralgia and right hypochondralgia. Initial examination revealed a fist-size mass at the epigastric fossa. Ultrasonography showed a hemangioma and a mosaic echoic lesion in the ventromedian with poor blood-flow signal and linear hyperechoic part inside, and a clear border to the surroundings. Dynamic computed tomography revealed a highly enhanced effect from the portal-venous phase continuing to the equilibrium phase. T1-weighted gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced image revealed a high intensity effect at the early phase that continued to the next phase. On the other hand, it contained a low intensity area by a fat suppression of that image. In addition, a T2-weighted image did not show a high intensity effect. Laparotomy was performed on the second day of hospitalization. The tumor had arisen from the ligamentum teres of the liver, and no metastasis or invasion of other organs was noted. It consisted of a lipid component of mature adipocytes and a fibrous component of deep dyeing pleomorphic or multinuclear atypical stromal cells. Immunohistochemical study of the atypical stromal cells demonstrated that they were positive for MDM2 and CDK4. A pathological diagnosis of atypical lipomatous tumor (ALT) was made, and the patient was discharged on the eighth day following the procedure. At the 6-mo follow-up dynamic CT, the patient was free of recurrence or metastasis. We experienced a patient with ALT in the ligamentum teres of the liver. This case suggests the need for a careful and detailed examination when encountering patients presenting with a mass; when neoplastic lesion is confirmed by image inspection, we should thoroughly investigate, including further image investigations and pathologic examination. The latter is the most important.
Core tip: Liposarcoma is one of the most common adult soft tissue sarcomas, accounting for approximately 20% of all mesenchymal malignancies. Atypical lipomatous tumor (ALT) is the most common intra-abdominal primary sarcomas. On the other hand, it is an extremely rare malignant adipose mesenchymal tumor. We report the first case of ALT occurring in the ligamentum teres of liver.
- Citation: Usuda D, Takeshima K, Sangen R, Nakamura K, Hayashi K, Okamura H, Kawai Y, Kasamaki Y, Iinuma Y, Saito H, Kanda T, Urashima S. Atypical lipomatous tumor in the ligamentum teres of liver: A case report and review of the literature. World J Clin Cases 2018; 6(12): 548-553
- URL: https://www.wjgnet.com/2307-8960/full/v6/i12/548.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i12.548
Liposarcoma is one of the most common adult soft tissue sarcomas, accounting for approximately 20% of all mesenchymal malignancies[1-3]. Well-differentiated and dedifferentiated liposarcoma, especially the former is called atypical lipomatous tumor (ALT). It is the most common intra-abdominal primary sarcoma[4]. On the other hand, it is an extremely rare malignant adipose mesenchymal tumor[5]. Typically, it is locally aggressive and shows a tendency toward recurrence after surgical excision, despite the fact that it does not metastasize and very rarely dedifferentiates[2]. As far as we know, there have been no reported liposarcoma cases occurring from the ligamentum teres of the liver. Herein, we report a case with a review of the existing literature.
The patient was a 61-year-old male, referred to our hospital with a three-month history of persistent epigastralgia and right hypochondralgia. Patient medical history included transient ischemic attacks, hypertension and hyperlipidemia, for which he was being treated with aspirin, antihypertensive medication, and bezafibrate. He was diagnosed with hepatic hemangioma following complete medical check-up ten years prior to his visit to our hospital. Examination revealed a fist-sized mass at the epigastric fossa. Other findings were normal. Routine blood tests showed elevated low-density lipoprotein cholesterol, decreased creatine kinase, and abnormal glucose tolerance. Blood count, C-reactive protein, liver enzyme, tumor maker including carcinoembryonic antigen, carbohydrate antigen 19-9, a fetal-specific glycoprotein antigen, and soluble interleukin-2 receptor were all within normal limits. In addition, the patient was negative for hepatitis B, C and syphilis, as well as collagen diseases. Abdominal ultrasonography (GE Healthcare, LOGIQ E9 XDclear 2.0) showed two tumors located in the right posterior superior segment of liver and ventromedian. The former showed hyperechoic lesion, namely hemangioma, the latter showed mosaic echoic lesion with poor blood flow signal, as well as linear hyperechoic part inside and a clear border to the surroundings (Figure 1). Dynamic CT (GE Healthcare, Discovery CT750 HD) revealed a highly enhanced effect from the portal-venous phase, continuing to the equilibrium phase for the latter tumor. MRI (GE Haelthcare, Signa HDxt 1.5T), including fat suppression radiography to confirm the existence of a lipid component, was performed. T1-weighted gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced imaging revealed a high intensity effect at the early phase that continued to the next phase. In addition, it showed a partial low intensity area by a fat suppression image (yellow arrow). On the other hand, a T2-weighted image did not show a highly enhanced effect (Figure 2). Absence of metastasis was confirmed. Gallium-67 scintigraphy (Canon Medical Systems, E.CAM) showed that there was no abnormal accumulation to the abdominal tumor.
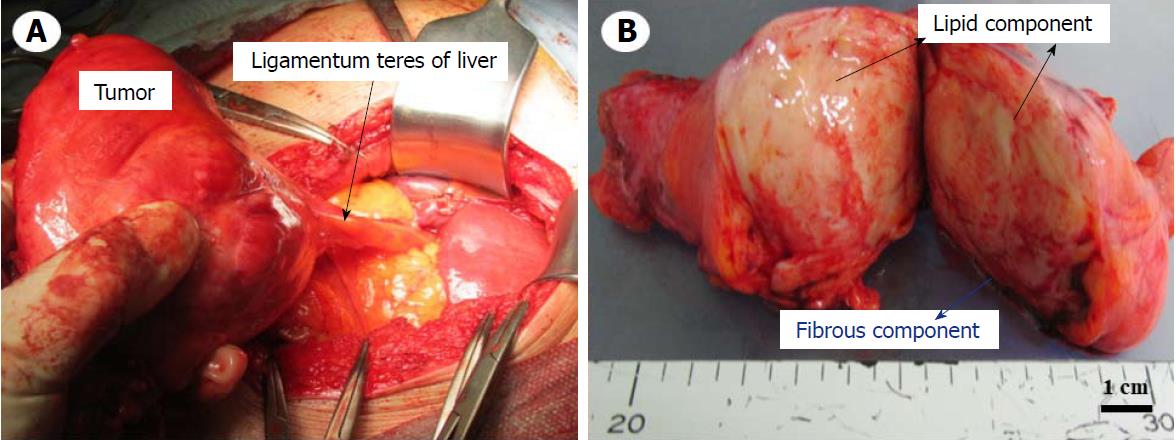
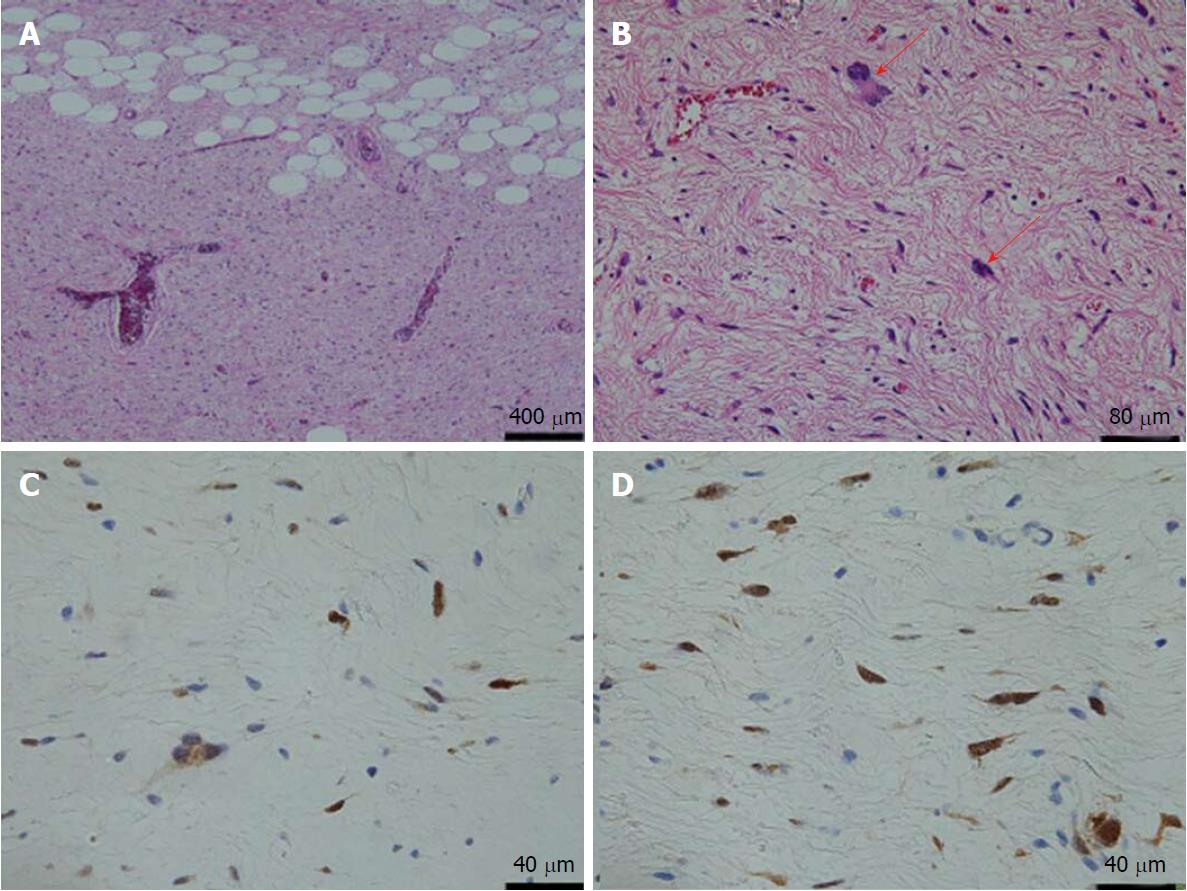
The above findings prompted a diagnosis of abdominal mesenchymoma, and we admitted the patient to the hospital. Laparotomy was performed on the second day post-admission. During an operation, we paid attention to two points for completing the tumor removal, avoiding bleeding and reducing the risk of recurrence; one was to apply gauze for discriminating between the tumor and surrounding tissues, and the other was to finely ligate surrounding blood vessels and cut them little by little. Intraoperative findings revealed as follows: (1) the tumor was arisen from the ligamentum teres of the liver and was highly covered with a capsule without invasion, adhesion, or infiltration to surrounding tissues. Therefore, it was easily enucleated; and (2) the absence of metastasis or other organ invasion was confirmed (Figure 3A). In the resected split specimen, the tumor size measured 13 cm x 9 cm x 5 cm and consisted of yellow lipid and a white fibrous component (Figure 3B). The lipid component was composed of mature adipocytes, and deep dyeing pleomorphic or multinuclear atypical stromal cells were confirmed in the fibrous component (Figure 4A and B). Immunohistochemistry of atypical stromal cells was positive for MDM2 and CDK4 and negative for α-SMA, S-100 protein, CD34 and STAT-6 (Figure 4C and D). Based on these findings, a pathological diagnosis of atypical lipomatous tumor (ALT) was made. The patient responded well and was discharged on the eighth day post-procedure. At the 6-mo follow-up dynamic CT appointment, the patient was free of recurrence or metastasis.
This is the first case of liposarcoma occurring from the ligamentum teres of the liver. As laid out in the World Health Organization’s classification of tumors of soft tissue and bone: IARC Press 2013, liposarcomas are divided into five subtypes: myxoid, pleomorphic, dedifferentiated, mixed liposarcoma, and ALT[5]. ALT is an intermediate tumor with risk of local recurrence but no potential for metastasis[1,5,6]. The points of differential diagnosis are focused on those mentioned above, especially dedifferentiated liposarcoma. However, it can be difficult to distinguish ALT from poorly differentiated sarcomas and benign adipose tumors[7]. The clinical presentation of ALT tends to be a progressive mass that can cause symptoms that are aesthetic, functional, or compressive in nature, depending on its size and localization[2].
On the other hand, chromosomal translocations and fusion genes are very commonly found in human cancer, particularly in liposarcoma and other subtypes of sarcomas[8]. The advancement of next-generation sequencing technologies, such as whole genome sequencing, has made it possible to discover novel chromosomal translocations and fusion genes in different tumors[8]. Recently, these next-generation sequencing approaches have led to the identification of many novel chromosomal translocations and gene fusions in different types of sarcomas[8]. In addition to sarcoma fusion genes that had previously been discovered, these novel specific fusion genes and their associated molecular events represent important targets for novel therapeutic approaches to sarcoma treatment[8]. Of these, the majority of ALTs express MDM2 (97%) and CDK4 (92%) on chromosome 12q13-15, compared to benign adipose tumors (MDM2, 5%; CDK4, 2%)[7,9]. The sensitivity and specificity of MDM2 and CDK4 immunostainings used to identify ALT among other soft tissue tumors were 97% and 92%, and 83% and 95%, respectively[7]. Therefore, MDM2 and CDK4 immunostaining is especially useful in separating ALT from large groups of differentiated adipose tumors, and this is important for the differential diagnosis[7]. In this case, we finally diagnosed ALT based on the immunostaining findings, which were positive for MDM2 and CDK4. In addition, there were also caveolae, which are cholesterol-enriched invaginations of the plasma membrane that are involved in various processes, including the absorption of glucose and fatty acids, cell transduction, and mechanoprotection[10]. In vitro analysis shows that both the biogenesis and the function of caveolae are dependent on the activity of the Caveolin (Cav-1, -2 and -3) and Cavin (Cavin-1, -2, -3 and -4) protein families[10]. Cavin-2, along with Cavin-1, Cav-1, and Cav-2, is mainly expressed in ALT, while it is almost impossible to detect in the more aggressive myxoid, pleomorphic tumors, and ALT[10]. In addition, the expression of Cavin-2 increases in liposarcoma tumor cell lines during differentiation, as opposed to proliferation in immunoblotting and immunofluorescence analysis[10]. Therefore, Cavin-2 serves as a useful marker that can be used to discriminate the degree of differentiation in liposarcoma tumors[10].
More studies are still necessary to determine the treatment and therapeutic strategies needed to improve the survival rate of patients with liposarcoma, as the disease is generally associated with frequent relapse[11]. To date, surgical resection has been the mainstay of curative treatment[11]. Several authors have recommended using wide excision with free margins in order to minimize the risk of recurrence, while others have reported having good results and a low rate of recurrence when opting for more conservative or even marginal excision, thereby avoiding complications caused by surgical site morbidity[2]. In fact, marginal excision is a good alternative in cases where the tumor is located near vascular or nerve structures, and it is not associated with elevated recurrence[2]. For metastatic disease, systemic treatment options have historically been represented by standard cytotoxic chemotherapy[3]. Eribulin has recently been approved for advanced liposarcoma, after anthracycline-containing regimen demonstrated an overall survival advantage in liposarcoma in a randomized Phase III clinical trial. However, further studies are required to better understand the precise mechanism of action of this agent and its potential role in combination schedules[12]. On the other hand, recent innovative therapies have been introduced and are currently part of the therapeutic armamentarium positively impacting disease control and patient quality of life[3]. Moreover, a better understanding of the molecular characteristics of each soft tissue sarcoma subtype over the last decade has allowed the detection of new potential targets and the development of novel, biology-driven compounds at different stages of testing[3]. Peculiar molecular features and fundamental signaling pathways now represent druggable targets for novel therapies[3]. Therapy that combines both CDK4 and RTK inhibitors may prove to be an effective option for ALT patients with RTK gene amplification[9]. In addition, liposarcoma subsets are less mutated, but they do express immunogenic self-antigens; as a result, strategies to improve antigen presentation and T-cell infiltration may potentially allow for successful immunotherapy in patients who have been given these diagnoses[13].
Long survival is correlated with the active resection of recurrence and recognition of high-grade dedifferentiated type liposarcoma at an early stage[10,11]. In addition, according to recent basic research, although limited compared to other malignancies, formalin-fixed paraffin-embedded tissue biomarkers, namely microRNA-155, may be novel independent indicators of unfavorable prognosis in liposarcoma[14]. However, there remains a need to identify predictive biomarkers for the better selection of target population and testing of combinations of drugs with the ultimate goal of improving outcomes[3].
In conclusion, we experienced a patient with ALT occurring in the ligamentum teres of the liver. This case suggests the need for a careful and detailed examination when encountering patients presenting with a mass; when neoplastic lesion is confirmed by image inspection, we should thoroughly investigate, including further image investigations and pathologic examination. The latter is especially important.
A three-month history of persistent epigastralgia and right hypochondralgia.
Abdominal tumor.
Neoplastic etiology.
Routine blood tests showed elevated low-density lipoprotein cholesterol, decreased creatine kinase, and abnormal glucose tolerance.
Abdominal mesenchymoma based on MRI, including of fat suppression radiography.
Immunohistochemical study of atypical stromal cells, namely tumor cells were positive for MDM2 and CDK4 and negative for α-SMA, S-100 protein, CD34 and STAT-6, which led to the pathological diagnosis of atypical lipomatous tumor (ALT).
Surgery.
ALT is an intermediate tumor with risk of local recurrence but no potential for metastasis. However, ALT may be difficult to distinguish from benign adipose tumors and poorly differentiated sarcomas. Up to now, MDM2 and CDK4 immunostaining have been particularly useful in separating ALT from the large group of differentiated adipose tumors, and they are important for the differential diagnosis. To date, surgical resection has been the mainstay of curative treatment. Long survival is correlated with the active resection of recurrence and recognition of high-grade dedifferentiated type liposarcoma at an early stage. As far as we know, there have been no reported ALT cases occurring from the ligamentum teres of the liver.
ALT: Atypical lipomatous tumor.
When neoplastic lesion is confirmed by image inspection, we should thoroughly investigate, including further image investigations and pathologic examination. The latter is especially important.
We thank Dr. Katsuaki Sato, from Department of Pathology II, Kanazawa Medical University, Japan, for pathological diagnosis of this case.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Guan YS, Kai K, Rong G S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Tan WW
| 1. | Dei Tos AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4:252-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Arvinius C, Torrecilla E, Beano-Collado J, García-Coiradas J, García-Maroto R, Puerto-Vázquez M, Cebrián-Parra JL. A clinical review of 11 cases of large-sized well-differentiated liposarcomas. Eur J Orthop Surg Traumatol. 2017;27:837-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Saponara M, Stacchiotti S, Gronchi A. Pharmacological therapies for Liposarcoma. Expert Rev Clin Pharmacol. 2017;10:361-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Levy AD, Manning MA, Al-Refaie WB, Miettinen MM. Soft-Tissue Sarcomas of the Abdomen and Pelvis: Radiologic-Pathologic Features, Part 1-Common Sarcomas: From the Radiologic Pathology Archives. Radiographics. 2017;37:462-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Sinaa M. [An exceptional association between retroperitoneal dedifferentiated liposarcoma and well differentiated pericolonic liposarcoma: about a case]. Pan Afr Med J. 2016;25:254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 603] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 7. | Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I, Coindre JM. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29:1340-1347. [PubMed] [Cited in This Article: ] |
| 8. | Xiao X, Garbutt CC, Hornicek F, Guo Z, Duan Z. Advances in chromosomal translocations and fusion genes in sarcomas and potential therapeutic applications. Cancer Treat Rev. 2018;63:61-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Asano N, Yoshida A, Mitani S, Kobayashi E, Shiotani B, Komiyama M, Fujimoto H, Chuman H, Morioka H, Matsumoto M. Frequent amplification of receptor tyrosine kinase genes in welldifferentiated/ dedifferentiated liposarcoma. Oncotarget. 2017;8:12941-12952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Codenotti S, Vezzoli M, Poliani PL, Cominelli M, Monti E, Fanzani A. Cavin-2 is a specific marker for detection of well-differentiated liposarcoma. Biochem Biophys Res Commun. 2017;493:660-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Dalal KM, Antonescu CR, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008;97:298-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Setola E, Noujaim J, Benson C, Chawla S, Palmerini E, Jones RL. Eribulin in advanced liposarcoma and leiomyosarcoma. Expert Rev Anticancer Ther. 2017;17:717-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, Redman MW, Baker KK, Cooper S, Donahue B. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123:3291-3304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Kapodistrias N, Mavridis K, Batistatou A, Gogou P, Karavasilis V, Sainis I, Briasoulis E, Scorilas A. Assessing the clinical value of microRNAs in formalin-fixed paraffin-embedded liposarcoma tissues: Overexpressed miR-155 is an indicator of poor prognosis. Oncotarget. 2017;8:6896-6913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |