12 December 2023: Original Paper
Short-Term Monitoring of Graft Regeneration in Partial Liver Transplantation Recipients
Yuying Shan1AB, Xi Yu1BC, Yuhao Du1BE, Jiongze Fang1BC, Jing Huang1EF, Jiangnan Sun1CDE, Yong Yang1DF, Shuqi Mao1BCD, Shengdong Wu1EF, Caide Lu1AF*DOI: 10.12659/AOT.941444
Ann Transplant 2023; 28:e941444
Abstract
BACKGROUND: Liver regeneration after partial liver transplantation, including living donor liver transplantation and split liver transplantation, is important for successful transplantation.
MATERIAL AND METHODS: We retrospectively analyzed 68 patients who underwent partial liver transplantation and calculated their regeneration index (RI)-based difference in postoperative and preoperative liver volume. We collected clinical data of donors and recipients and analyzed the correlation between clinical characteristics and RI. According to the above results, the generalized estimating equation (GEE) model included white blood cell count (WBC), neutrophils, lymphocytes, platelets, prothrombin time (PT), and activated partial thromboplastin time (APTT) on Days 1, 3, and 7 after LT and was used to predict the RI.
RESULTS: The mean RI was 40%, which was used as the cutoff value to divide all patients to the high-RI group and the low-RI group. The percentage of Child-Pugh C patients was 44% in the high-RI group, which was significantly more than that (21%) in the low-RI group (P=0.038). Among the postoperative monitoring parameters, neutrophil (P=0.044) and platelet (P=0.036) levels declined in the high-RI group on Day 3, while APTT was higher on Day 1 compared to the low-RI group. The predictive model based on GEE analysis achieved a good effect, with the area under the receiver operating characteristic curve on Day 1 (0.681; 95% CI, 0.556-0.807) and Day 3 (0.705; 95% CI, 0.578-0.832) showing significant differences (P=0.010 and 0.004, respectively).
CONCLUSIONS: The combination of decreased counts of WBC, neutrophils, lymphocytes, and platelets, as well as elevated PT and APTT on Day 3 after LT showed a good capability to predict a higher rate of liver regeneration after partial liver transplantation.
Keywords: Liver Regeneration, Liver Transplantation, Longitudinal Studies
Background
Liver transplantation (LT) is the most effective treatment of end-stage liver disease. With the increasingly mature techniques of LT and continuous improvement in immunotherapy regimens, the survival rate of LT has gradually improved, along with increased benefits for patients. A pivotal problem is the persistent shortage of organ donations, resulting in many patients with end-stage liver disease deteriorating and even dying while waiting for a suitable donor liver. To alleviate the shortage of donor livers, more techniques such as living donor LT (LDLT), split LT (SLT), auxiliary LT, and reduced LT have gradually been developed. The development of these techniques can effectively expand the source of donor livers. In SLT, a complete donor liver is divided into 2 or more anatomical functional units, which are transplanted into 2 or more recipients. Basic split surgeries can be classified into classical SLT (cSLT) and full-size SLT (fSLT). The fSLT divides the whole liver into left and right lobes, which can be transplanted into 2 adults (or older children equivalent to adults) [1,2]. In cSLT, the whole liver was split into left lateral segment and right tri-segment graft, which are suitable for 1 child and 1 adult. In the present report we show that partial liver transplantation in adults, including LDLT and fSLT, can effectively improve the utilization of donor livers and alleviate the shortage of donor livers.
The biggest problem with fSLT is that the 2 half lobes need to meet the needs of 2 adults. In LDLT, the half lobe needs to meet the needs of recipients, while the remaining half lobe should ensure the safety of the donor. Thus, liver regeneration is critical for successful implementation of partial liver transplantation. If the volume is insufficient or regeneration is poor, the recipient will develop small-for-size syndrome, which seriously affects the prognosis [3,4]. Clinical studies have demonstrated that the regenerative ability after transplantation has significant individual differences due to the complex conditions of recipients. Thus, accurate estimation of the regenerative ability after surgery is necessary to avoid postoperative liver insufficiency and liver failure.
Many cytokines, growth factors, and signaling pathways work together to promote liver regeneration. Most studies on the mechanism of liver regeneration used partial hepatectomy animal models; however, there are differences between hepatectomy and liver transplantation due to hemodynamic changes in the portal venous flow or pressure, tissue ischemia/hypoxia, and hemostasis/platelet activation [5–8]. Therefore, the mechanism and clinical manifestations of liver regeneration after transplantation need to be further elucidated.
At present, there is no report on longitudinal monitoring of serum indicators after liver transplantation, and the assessment of liver regeneration mostly relies on direct evaluation of clinicians based on computed tomography (CT), magnetic resonance imaging (MRI), and other imaging data, which cannot accurately and dynamically monitor the regeneration ability. This study aimed to identify perioperative clinical factors that influence regeneration after liver transplantation. Additionally, we attempted to predict liver regeneration after partial LT using common postoperative laboratory indicators.
Material and Methods
PATIENTS AND DONORS:
From May 2018 to September 2022, 72 consecutive patients underwent partial LT, including 17 LDLTs and 55 fSLTs, at Ningbo Medical Center Lihuili Hospital. A total of 4 recipients died within the first month after LT; the cause of death included graft-versus-host disease (n=1), portal vein thrombosis (n=1), and severe sepsis (n=2). After excluding 4 perioperative death recipients, a total of 68 recipients including 14 LDLTs and 54 fSLTs recipients (grafts from 32 donors) were enrolled in this study. Other donated grafts were allocated to other medical centers. The details of the patient selection procedure are shown in Figure 1.
Donors: According to previous research [9–11] and our experiences, all the donors in this research met the criteria: (1) hemodynamically stable with acceptable cardiopulmonary function; (2) no uncontrollable bacterial infection, and no malignant neoplasms; (3) age ≤60 years old; (4) body weight ≥50 kg; (5) body mass index (BMI) ≤25 kg/m2; (6) the pathology showed fatty liver ≤0% or no fatty liver by biopsy; (7) no clear systemic infection; (8) stable hemodynamics or taking a small number of vasoactive drugs; (9) alanine transaminase (ALT)/ aspartate transaminase (AST) <3 times the normal value and total bilirubin ≤2 times the normal value; (10) and cardiopulmonary resuscitation ≤1 time. All the SLT donors were donors of brain death (DBD). When the DBD donor met all the above criteria, CT was performed before donation. If three-dimensional (3D) reconstruction based on CT images showed no malformation of the blood vessels and bile ducts and ensured good blood supply of grafts after splitting, and the left and right half liver volumes could meet the required graft-to-recipient weight ratio (GRWR) of the matched recipient ≥0.8%, then the doctor who obtained the liver during the operation would determine whether splitting can be performed by observing the appearance, color, texture, infusion situation, and the vascular and bile duct conditions, combined with intraoperative biopsy. All of the SLTs were in situ fSLTs that separated the whole liver into the half right lobe and half left lobe to meet the need of 2 adults. All SLT recipients were allocated to the Chinese Organ Transplant Response System (COTRS; https://www.cot.org.cn/). All donations were approved by the ethics committee.
Recipients: Recipients who had been evaluated and included in the waiting list for liver transplantation had to meet the following criteria: (1) Model for End-Stage Liver Disease (MELD) score <30; (2) no re-LT; (3) no more than 1 liver surgery; (4) estimated liver segment volume (ml)/recipient body weight (g) or GRWR ≥0.8%; (5) no severe portal hypertension; (6) no grade 1–2 portal vein/superior mesenteric vein thrombosis; (7) no severe vascular variation. All the recipients were adults.
All medical records of donors and recipients were retrospectively reviewed, and data were collected for analysis with the approval of the Institutional Review Board of Ningbo Medical Center Lihuili Hospital (approval no. KY2022PJ019). The need for informed consent from the patients was waived because of the retrospective nature of the study. The study protocol complied with the ethical standards of the Declaration of Helsinki with respect to patient data confidentiality. No organs from prisoners were used in this study.
CT VOLUMETRY PROTOCOL AND GRAFT VOLUME MEASUREMENT:
All the donors and recipients underwent contrast-enhanced CT (CECT) examinations using the same CT scanner (Philips Ingenuity Flex, Amsterdam, Netherlands) and the images were saved in the Digital Imaging and Communications in Medicine (DICOM) format. The DICOM files were sorted using Mimics™ 19 software (Materialise, NV, Belgium). The software automatically recognizes the imported CT images and generates 3 interrelated views in the sagittal plane, coronal plane, and cross-section. A segmentation tool was used to perform threshold segmentation on images from different vascular phases of CECT (the segmentation threshold was 20–200 Heinz units). A region-growing tool was used to form the 3D masks of the liver. The mask was then edited to fill in the gaps and holes in the structure. Finally, the necessary cutting and smoothing processes were performed according to the anatomy, and the matching positions were adjusted to build the 3D model of the liver, which was saved in stereolithography format. Parameters such as the total liver volume and left and right lobe volumes were automatically calculated and generated using the Mimics19.0 software query module. The examples of images and models are shown in Figure 2.
A CT examination was performed before the donor liver was obtained, and the whole-liver volume and left and right half-lobe volumes of the donor liver were calculated (LVpre). The recipient underwent CT 1 month after LT, when the postoperative volume was calculated (LVpost). The regeneration index (RI) was calculated using the following equation: RI=(LVpost–LVpre)/LVpre×100 (%).
DATA COLLECTION AND STATISTICAL ANALYSIS:
We collected the following perioperative clinical data. Donors: age, sex, body mass index (BMI), body surface area (BSA), whole-liver weight, graft weight, standard liver volume (SLV), estimated total liver volume, estimated graft volume, graft weight, graft volume at one month after LT, graft type, and cold ischemia time. Recipients: age, sex, BMI, BSA, GRWR, MELD score, hepatitis B virus (HBV) infection, presence of malignancy, duration of operation, anhepatic phase, intraoperative blood loss and blood transfusion, postoperative complications, and hospital stay length. During postoperative monitoring, we selected indicators from among the routine examination items, including routine blood tests, liver function, and coagulation function on Days 1, 3, and 7 after LT, which has the advantages of being simple and fast, without imposing an additional burden on recipients.
Mosteller’s formula was used for calculating the BSA: BSA (m2)=[Height (cm)×Weight (kg)/3600][12].
We calculated the SLV as: SLV (ml)=706.2×BSA (m2)+2.4 [13].
Pearson’s chi-square test or Fisher’s exact test was used to compare the frequencies of categorical variables between the high-RI and low-RI groups. The independent samples
Results
BASELINE CHARACTERISTICS:
A total of 68 liver transplant recipients (14 LDLT and 54 SLT) were enrolled in this study. Sixty-eight grafts were obtained from 14 LDLT donors and 34 DBD donors. The recipients were predominantly men (75%), the average age was 53±11 years, average BMI was 21.7±3.1 kg/m2, and average SLV was 1175±124 ml. The characteristics of the recipients and donors are summarized in Table 1. The median RI was 40% (0–361%), which was used to separate the patients into low- and high-RI groups. The average volumes calculated by 3D reconstruction of the initial donor liver segment were 813±233 ml and 732±186 ml, and the average liver volumes in the first month after the operation were 968±294 ml and 1223±259 ml in the low and high-RI groups, respectively.
Compared with the low-RI group, the high-RI group recipients had a younger average age (50±11 vs 55±10 years), higher percentage of men (85.3% vs 64.7%), higher percentage of right graft (58% vs 42%), lower percentage of left graft (41% vs 59%), lower percentage of HBV infection (56% vs 68%), and lower percentage of malignancy (47% vs 59%). However, the differences in the above parameters were not statistically significant. Additionally, the graft weights were 718±216 g and 705±219 g, GRWR was 1.2±0.3% and 1.1±0.3%, the number of left grafts was 19 and 13, and number of right grafts was 15 and 21 in the low- and high-RI groups, respectively. The percentage of Child-Pugh C patients was 44% in the high-RI group, which was significantly more than the 21% in the low-RI group (P=0.038). There were no significant differences in graft weight, GRWR, operation time, graft type, BMI, HBV infection, tumor, postoperative intensive care unit (ICU) stay, or postoperative hospital stay between the high- and low-RI groups. The details are listed in Table 2.
POSTOPERATIVE MONITORING MODEL:
On analyzing the postoperative factors on Days 1, 3, and 7 after transplantation, the factors showed different relationships with RI at different time points. Neutrophil (P=0.044) and platelet (P=0.036) levels decreased in the high-RI group on Day 3. APTT was higher on Day 1 in the high-RI group, but decreased to the same level as that in the low-RI group. We did not find significant differences in other laboratory factors, such as total bilirubin, albumin, AST, and ALT levels, between the high- and low-RI groups. The associations of white blood cells (WBC) count, neutrophil count, lymphocyte count, platelet count, prothrombin time (PT), and activated partial thromboplastin time (APTT) separately with the high- and low-RI groups in the GEE analysis are shown in Table 3. In the GEE analysis, WBC count, neutrophil count, lymphocyte count, platelet count, PT, and APTT were included in the model on Days 1, 3, and 7, as shown in Figures 3, 4 and Table 4. The area under the receiver operating characteristic curve (AUROC) values for the model on Day 1 (0.681; 95% confidence interval [CI], 0.556–0.807) and Day 3 (0.705; 95% CI, 0.578–0.832) showed significant differences (P=0.010 and 0.004, respectively), as shown in Figure 5 and Table 5.
Discussion
In our data, the RI ranged from 0% to 361%, which showed high variability. Younger, men, Child-Pugh C recipients, and right grafts lead to better regeneration capacity. We established a model consisting of white blood cell count, neutrophils, lymphocytes, platelets, prothrombin time, and activated partial thromboplastin time on Days 1 and 3 after liver transplantation to monitor and predict the liver transplantation outcomes.
In recent years, 3D reconstruction has been widely used for the preoperative evaluation of liver resection. Volumetric CT plays an important role in evaluating liver volume because it has good reconstructive ability [14–16]. The application of 3D reconstruction technology to split the donor liver helps in accurate segmentation of the split liver, as well as accurate liver cutting and volume calculations. Therefore, we applied 3D reconstruction to every donor liver to evaluate the whole-liver volume and each segment’s volume, which facilitated liver splitting.
Previous studies on liver regeneration after partial hepatectomy in LDLT have demonstrated the high regenerative potential of liver remnants in donors and the safety of liver donation [17–24]. It was reported that liver regeneration was triggered immediately after resection [25], and the regeneration rate tends to be the most rapid for 1 month, slows down after 1 month, and stops after 6 months [26]. Thus, we chose 1 month after the operation as the observation cutoff point to calculate the difference between the calculated liver volume and donor liver volume. In our study, the median regeneration rate was 40%, which is consistent with that reported by Fausto [25,26]. Humar reported that by 3 months after transplantation, most patients had almost doubled their liver size, and the liver volume increased by a mean of approximately 2.2-fold in recipients [27]. These results confirmed that the regeneration rate was the fastest 1 month after transplantation and slowed in the next 3 months, until it stopped. By analyzing the correlation between perioperative indicators and the liver regeneration rate, we found that Child-Pugh grade C was correlated with a higher RI, indicating that liver failure could accurately predict liver regeneration. In the high-RI group, the average age of the recipients was younger (50 vs 55 years) and the proportion of men was higher (85.3% vs 64.7%); however, graft weight, GRWR, cold ischemia time, graft type (left/right lobe), donor type (DDLT/LDLT), recipient sex, age, BMI, HBV, malignancy, operation time, anhepatic phase, operative blood loss, intraoperative blood transfusion, postoperative ICU stay, and hospital stay were not significantly correlated with liver regeneration. These results were similar to those of Humar et al, except that they reported that recipient and graft weights were positively associated with the liver regeneration rate. Humar et al also reported that donor-recipient pairs did not show any correlation in the regeneration parameters between the 2 parts of the same liver, indicating that the host plays a significant role in driving the process [27]. There were also some preoperative laboratory indicators related to liver regeneration. Park et al reported that female donors, female recipients, and higher preoperative WBC count and protein were correlated with a higher liver regenerate rate [28]. Zhang et al investigated liver regeneration in patients who underwent right hepatectomy and found that preoperative lower AST, higher albumin, and higher PT-INR were positively correlated with higher RI when the cutoff value of RI was 100% [14].
We monitored various laboratory indicators on Days 1, 3, and 7 after transplantation and found that the high-RI group exhibited lower neutrophil and platelet levels on the Day 3 (
Among the various factors reported to be associated with liver regeneration [31–33], platelets undoubtedly play an essential role. Several studies have demonstrated that a low platelet count after liver resection or partial liver transplantation is associated with poor liver regeneration and vice versa [34–36]. Lisman et al summarized the potential mechanisms underlying platelet-mediated liver regeneration [37]. Platelets rapidly migrate into the space of Disse and release their contents, stimulating hepatocyte or liver sinusoidal endothelial cell proliferation via HGF, IGF-1, VEGF, and serotonin [37,38]. In addition to hepatocytes, platelets interact with sinusoidal endothelial cells and Kupffer cells, thereby improving liver regeneration. Sphingosine-1-phosphate is secreted by platelets, which stimulates sinusoidal endothelial cell proliferation and IL-6 secretion, driving DNA synthesis in hepatocytes. The interaction between platelets and Kupffer cells leads to the activation of both cells [39]. Platelets enhance secretion of mediators, including TNF-α and IL-6, by Kupffer cells, which are required for liver regeneration [38]. We observed that platelets were expressed at significantly lower levels in the high-RI group than in the low-RI group. Furthermore, platelets in the high-RI group decreased immediately and then gradually increased, indicating that platelets were rapidly consumed when regeneration was activated after transplantation and then gradually returned to their original levels. Thus, we believe that platelets can be used as a potential monitoring tool for regeneration. Among the immune cells, macrophages, Kupffer cells, eosinophils, and T lymphocytes have been highlighted as the cells that affect liver regeneration, either through direct contact or via cytokine production. A few studies have investigated the relationship of lymphocytes and neutrophils with liver regeneration [39–42]. Since immune cells have been reported to play an essential role in liver regeneration, we believe that they can be monitored by laboratory tests.
There were several limitations of our study. First, selection bias may present because of the single-center and retrospective analysis. The results still need to be verified. Second, we could only observe 1-month postoperative graft volume, and relied on CT texture, but the actual graft weight was unknown. Third, as a clinical study, we could only discover the related indicators, but the specific mechanisms and related pathways still need to be elucidated.
Conclusions
In conclusion, we found that the combination of decreased WBC count, neutrophils, lymphocytes, and platelets, as well as elevated PT and APTT, on Day 3 after LT can predict rapid liver regeneration within the first month. Our results may greatly help clinicians monitor and predict regeneration ability in the planning stage to prevent unexpected liver failure after partial liver transplantation.
Figures
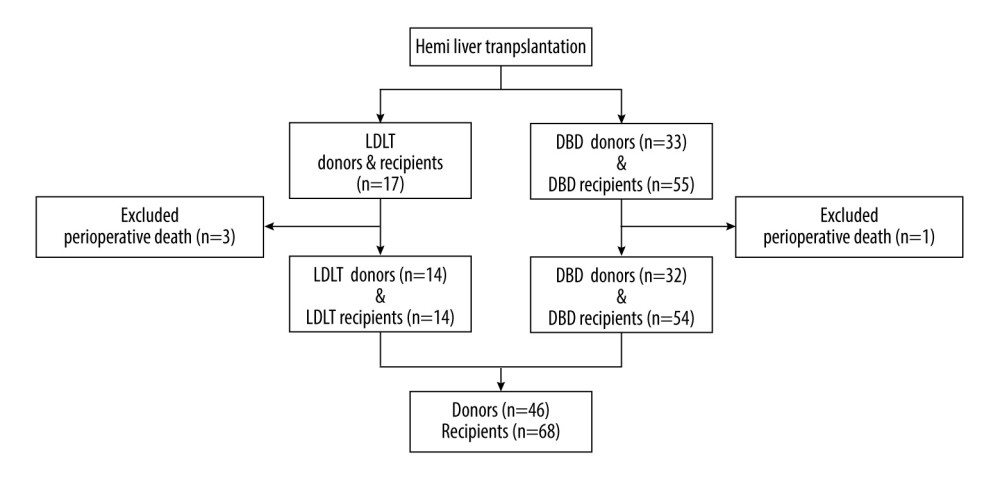 Figure 1. Flow chart of patient enrolment. (Created with PowerPoint 2016, Microsoft Corporation).
Figure 1. Flow chart of patient enrolment. (Created with PowerPoint 2016, Microsoft Corporation). 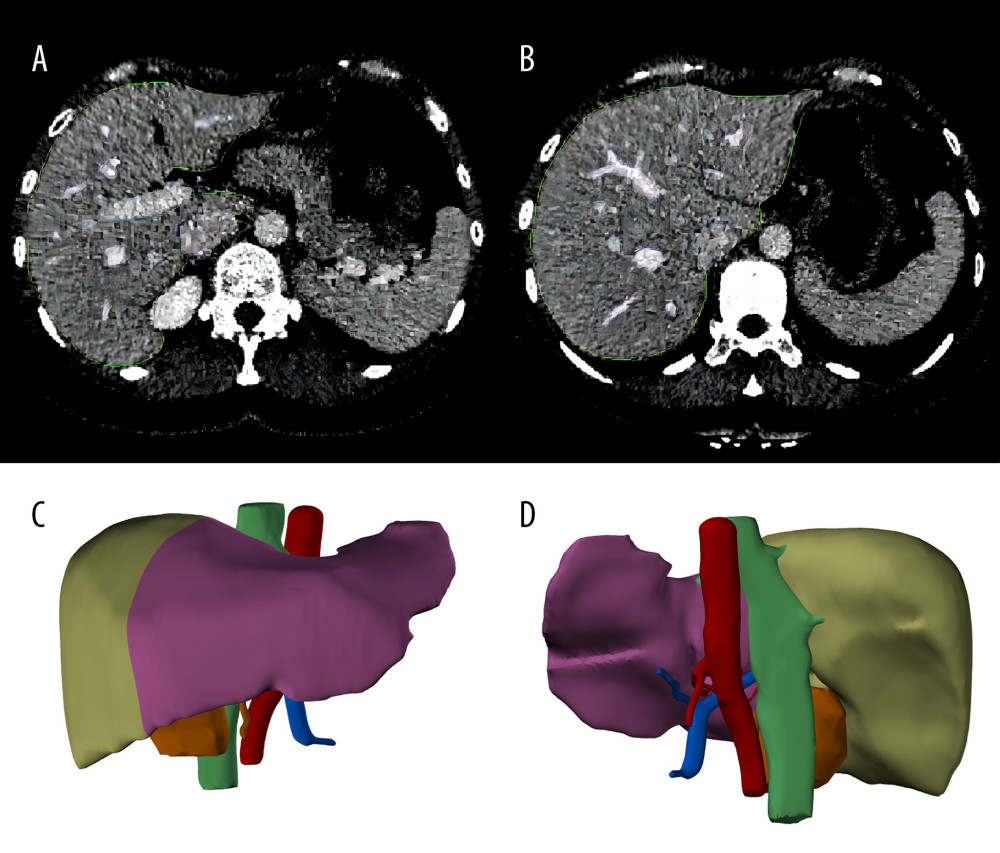 Figure 2. Liver segmentation using Mimics software program. (A, B), the liver was recognized in CT images. The green lines surround the scope of liver reconstruction. The purple and blue lines surround the hepatic veins and portal veins and their intrahepatic branches, respectively. (C, D), a whole liver was separated into left lobe (purple) and right lobe (light green), and the portal veins (blue), hepatic arteries (red) and biliary ducts (green) were reconstructed. (Created with Mimics 19.0 software)
Figure 2. Liver segmentation using Mimics software program. (A, B), the liver was recognized in CT images. The green lines surround the scope of liver reconstruction. The purple and blue lines surround the hepatic veins and portal veins and their intrahepatic branches, respectively. (C, D), a whole liver was separated into left lobe (purple) and right lobe (light green), and the portal veins (blue), hepatic arteries (red) and biliary ducts (green) were reconstructed. (Created with Mimics 19.0 software) 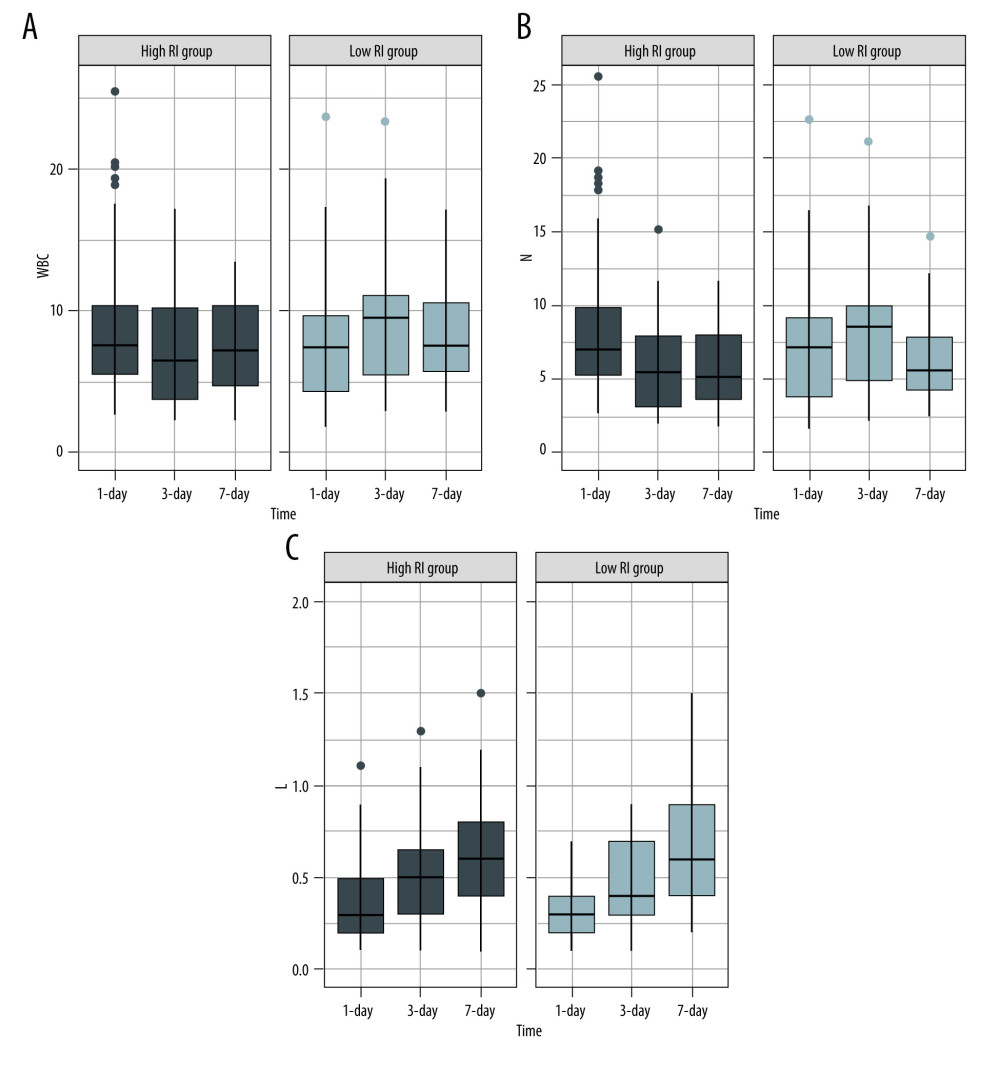 Figure 3. Serial changes in the serum inflammatory biomarker levels in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (A) White blood cell (WBC) count, (B) Neutrophils, (C) Leukocytes. (Created with R version 3.6.2)
Figure 3. Serial changes in the serum inflammatory biomarker levels in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (A) White blood cell (WBC) count, (B) Neutrophils, (C) Leukocytes. (Created with R version 3.6.2) 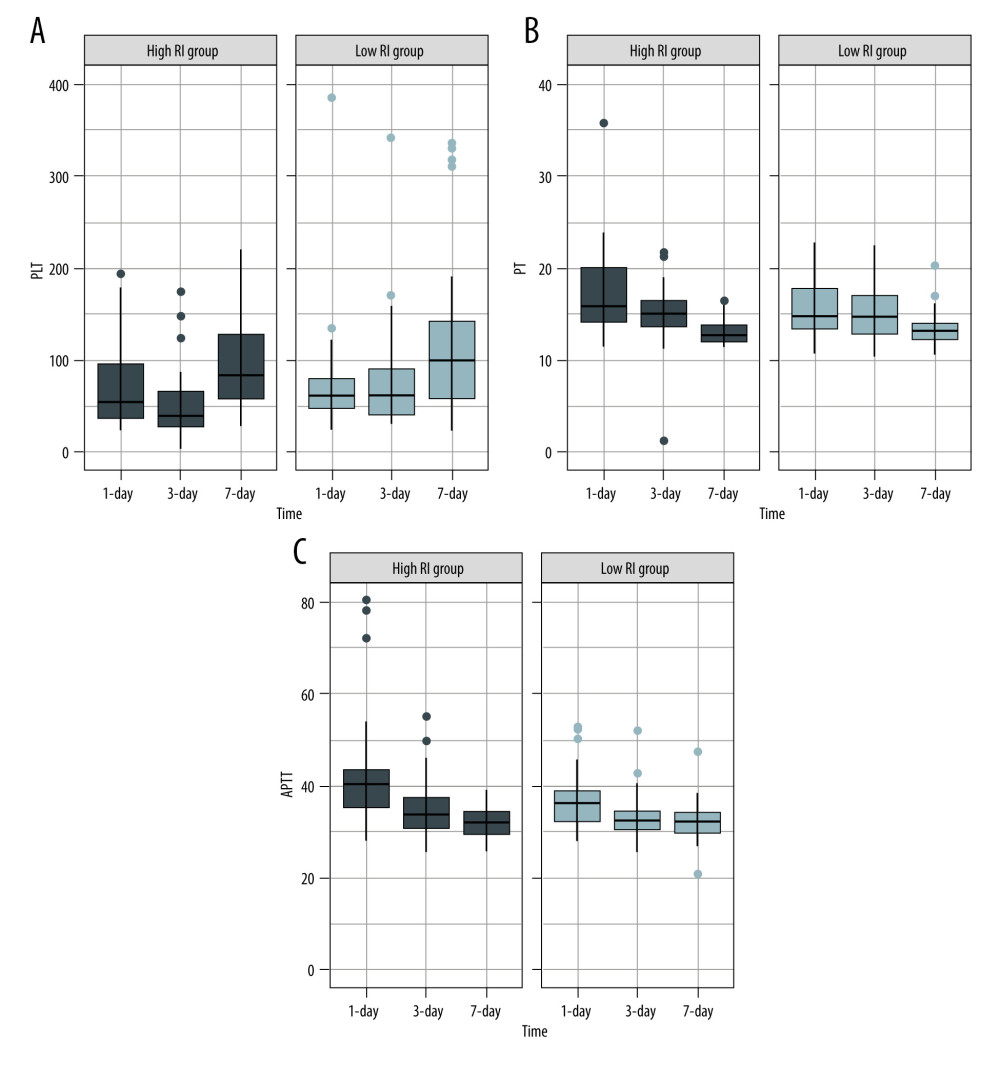 Figure 4. Serial changes in (A) platelets (PLT), (B) prothrombin time (PT), and (C) activated partial thromboplastin time (APTT) in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (Created with R version 3.6.2)
Figure 4. Serial changes in (A) platelets (PLT), (B) prothrombin time (PT), and (C) activated partial thromboplastin time (APTT) in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (Created with R version 3.6.2) 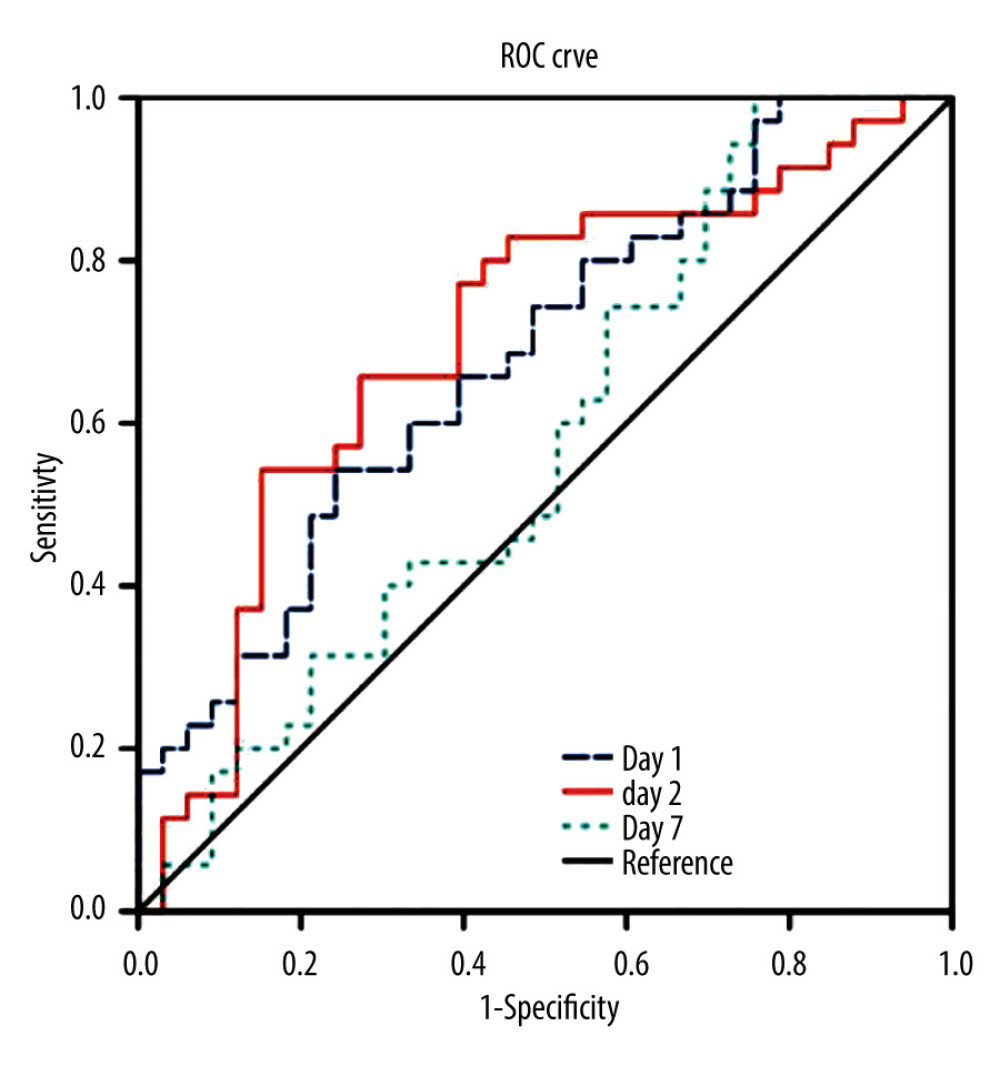 Figure 5. The area under the receiver operating characteristic curve (AUROC) for the combination of the biomarkers of white blood cell (WBC) count, neutrophils, leukocytes, platelets, prothrombin time (PT), and activated partial thromboplastin time APTT on Days 1, 3, and 7 after transplantation. (Created with SPSS version 25.0, IBM Corporation).
Figure 5. The area under the receiver operating characteristic curve (AUROC) for the combination of the biomarkers of white blood cell (WBC) count, neutrophils, leukocytes, platelets, prothrombin time (PT), and activated partial thromboplastin time APTT on Days 1, 3, and 7 after transplantation. (Created with SPSS version 25.0, IBM Corporation). Tables
Table 1. Characteristics of donors and recipients.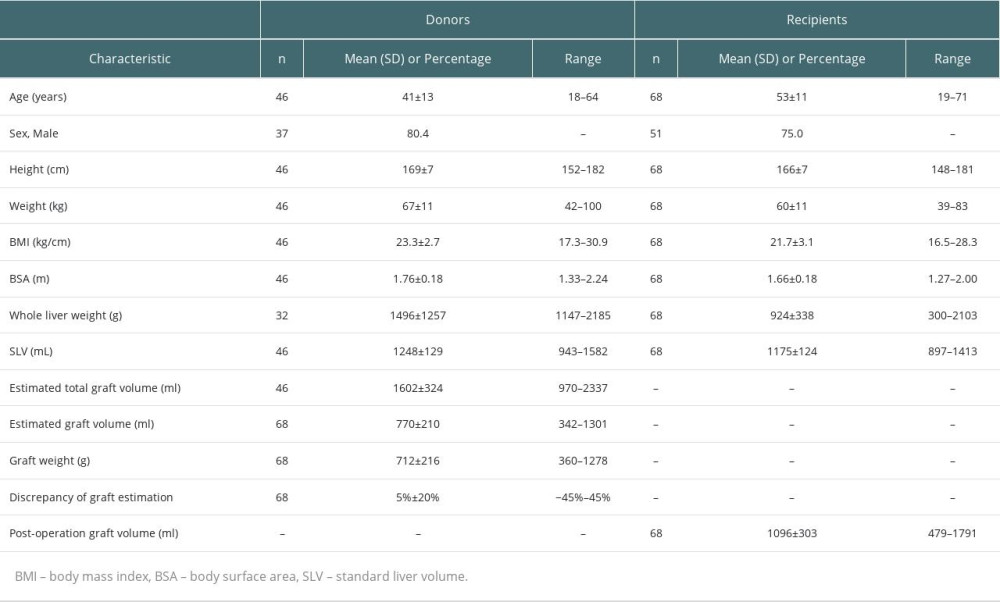 Table 2. Comparisons of characteristics of grafts and clinical parameters of recipients between low-RI and high-RI groups.
Table 2. Comparisons of characteristics of grafts and clinical parameters of recipients between low-RI and high-RI groups.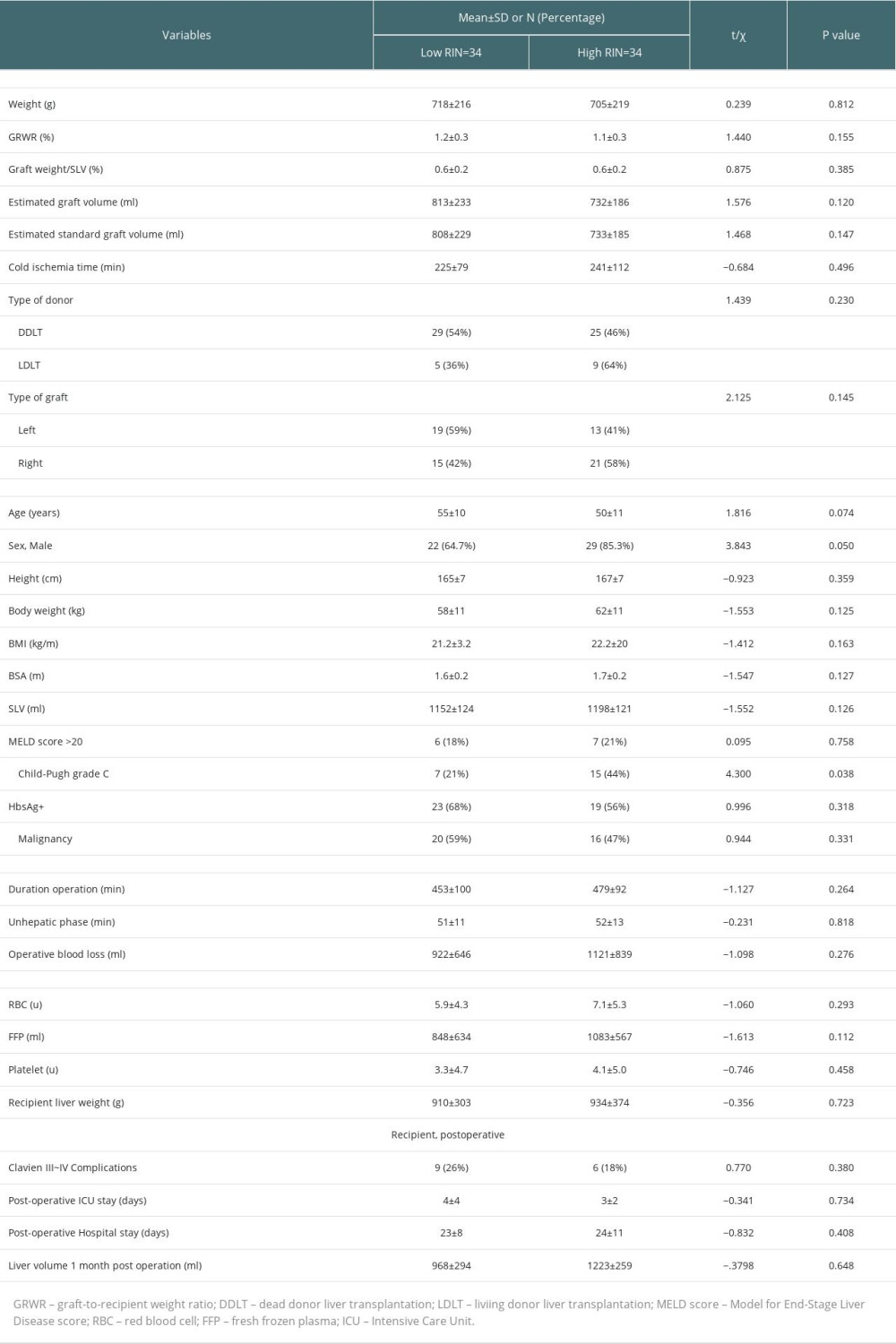 Table 3. Comparisons of postoperative laboratory parameters between high-RI and low-RI groups.
Table 3. Comparisons of postoperative laboratory parameters between high-RI and low-RI groups.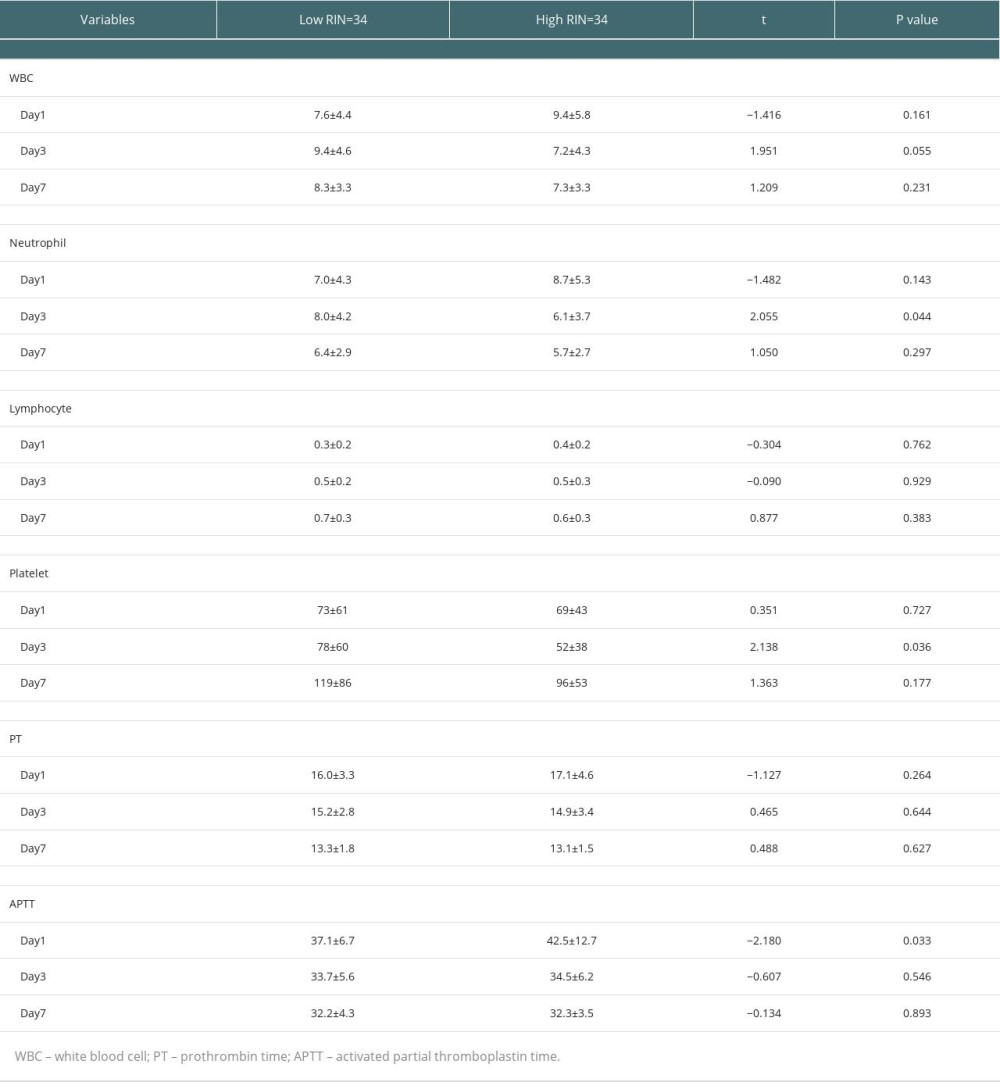 Table 4. Association of parameters between high-RI and low-RI groups in the GEE analysis.
Table 4. Association of parameters between high-RI and low-RI groups in the GEE analysis.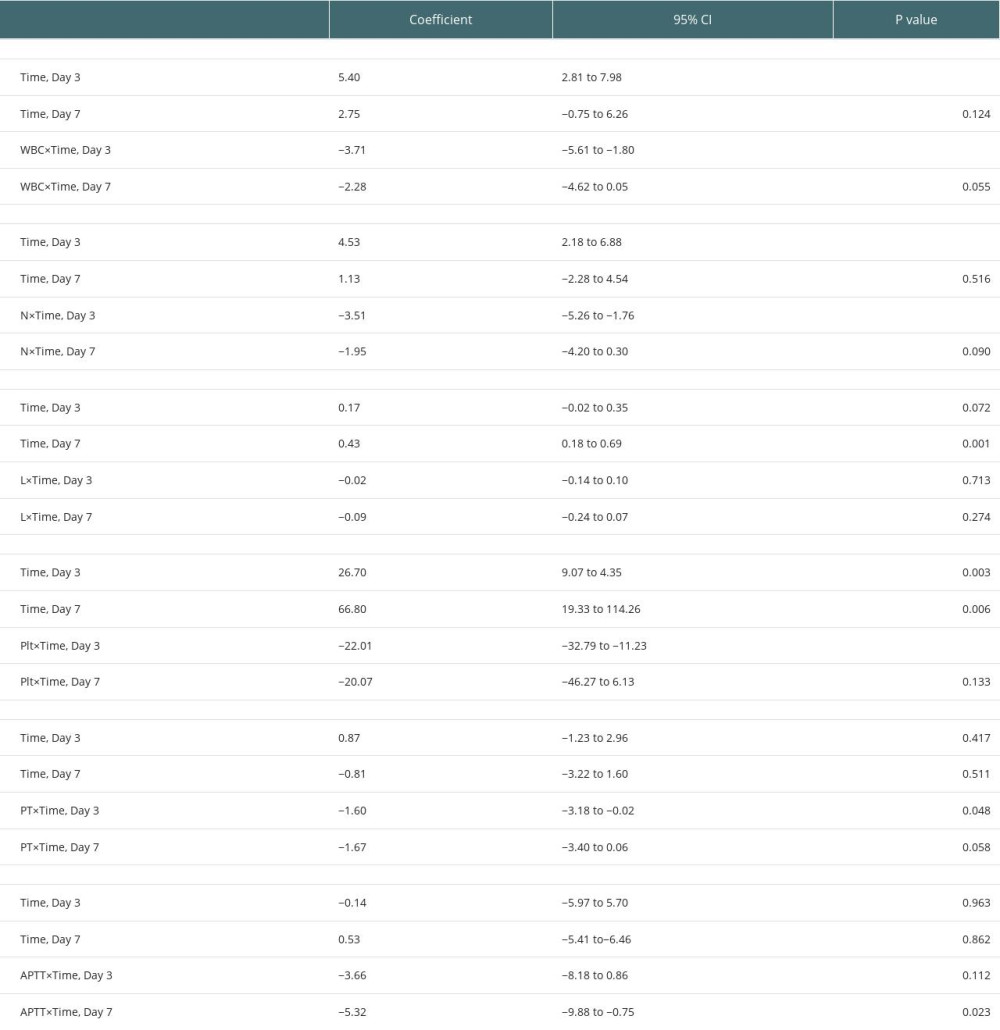 Table 5. AUROC for combination of WBC, neutrophil, lymphocyte, platelet, PT, and APTT on Days 1, 3, and 7.
Table 5. AUROC for combination of WBC, neutrophil, lymphocyte, platelet, PT, and APTT on Days 1, 3, and 7.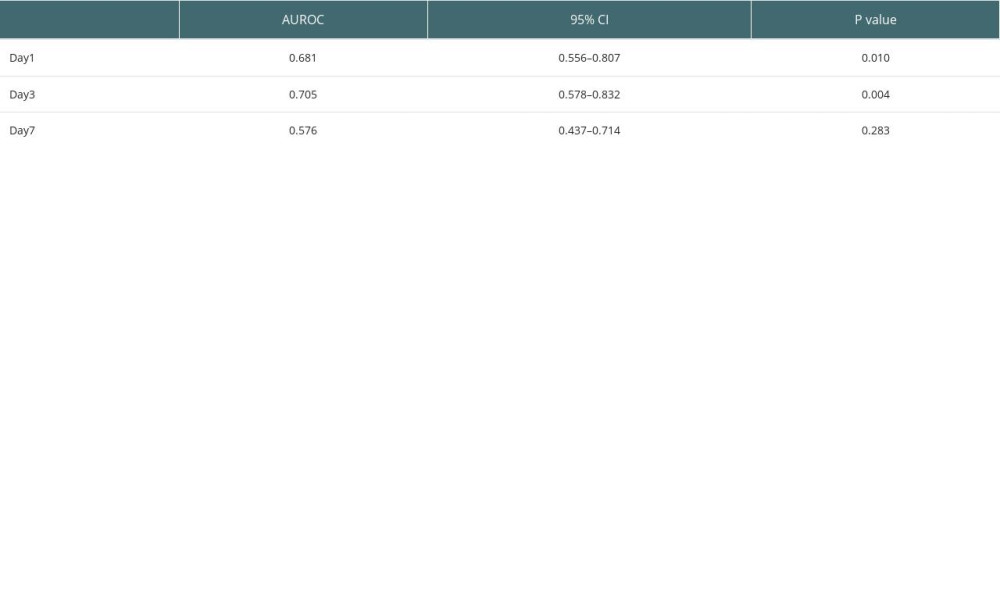
References
1. Busuttil RW, Goss JA, Split liver transplantation: Ann Surg, 1999; 229(3); 313-21
2. Azoulay D, Castaing D, Adam R, Split-liver transplantation for two adult recipients: Feasibility and long-term outcomes: Ann Surg, 2001; 233(4); 565-74
3. Vagefi PA, Parekh J, Ascher NL, Outcomes with split liver transplantation in 106 recipients: The University of California, San Francisco, experience from 1993 to 2010: Arch Surg, 2011; 146(9); 1052-59
4. Kong L, Lv T, Jiang L, Outcomes of hemi- versus whole liver transplantation in patients from mainland China with high model for end-stage liver disease scores: a matched analysis: BMC Surg, 2020; 20(1); 290
5. Gilgenkrantz H, De L’Hortet AC, Understanding liver regeneration: From mechanisms to regenerative medicine: Am J Pathol, 2018; 188; 1316-27
6. Van Haele M, Snoeck , Roskams T, Human liver regeneration: An etiology dependent process: Int J Mol Sci, 2019; 20; 2332
7. Ozaki M, Cellular and molecular mechanisms of liver regeneration: Proliferation, growth, death and protection of hepatocytes: Semin Cell Dev Biol, 2020; 100; 62-73
8. Shi J, Line PD, Hallmarks of postoperative liver regeneration: An updated insight on the regulatory mechanisms: J Gastroenterol Hepatol, 2019; 35; 960-66
9. Hackl C, Schmidt KM, Süsal C, Split liver transplantation: Current developments: World J Gastroenterol, 2018; 24(47); 5312-21
10. Hashimoto K, Quintini C, Aucejo FN, Split liver transplantation using Hemiliver graft in the MELD era: A single center experience in the United States: Am J Transplant, 2014; 14(9); 2072-80
11. Yerdel MA, Gunson B, Mirza D, Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome: Transplantation, 2000; 69(9); 1873-81
12. Urata K, Kawasaki S, Matsunami H, Calculation of child and adult standard liver volume for liver transplantation: Hepatology, 1995; 21(5); 1317-21
13. Mosteller RD, Simplified calculation of body-surface area: N Engl J Med, 1987; 317; 1098
14. Zhang T, Wei Y, He X, Prediction of remnant liver regeneration after right hepatectomy in patients with hepatocellular carcinoma using preoperative CT texture analysis and clinical features: Contrast Media Mol Imaging, 2021; 2021; 5572470
15. Hiroshige S, Shimada M, Harada N, Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography: Transplantation, 2003; 75(9); 1561-64
16. Nakagami M, Morimoto T, Itoh K, Patterns of restoration of remnant liver volume after graft harvesting in donors for living related liver transplantation: Transplant Proc, 1998; 30(1); 195-99
17. Marcos A, Fisher RA, Ham JM, Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation: Transplantation, 2000; 69(7); 1375-79
18. Pascher A, Sauer IM, Walter M, Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation: Liver Transpl, 2002; 8(9); 829-37
19. Kwon KH, Kim YW, Postoperative liver regeneration and complication in live liver donor after partial hepatectomy for living donor liver transplantation: Yonsei Med J, 2003; 44(6); 1069-77
20. Pomfret EA, Early and late complications in the right-lobe adult living donor: Liver Transpl, 2003; 9(10 Suppl 2); S45-49
21. Nadalin S, Testa G, Malagó M, Volumetric and functional recovery of the liver after right hepatectomy for living donation: Liver Transpl, 2004; 10(8); 1024-29
22. Ibrahim S, Chen CL, Wang CC, Liver regeneration and splenic enlargement in donors after living-donor liver transplantation: World J Surg, 2005; 29(12); 1658-66
23. Haga J, Shimazu M, Wakabayashi G, Liver regeneration in donors and adult recipients after living donor liver transplantation: Liver Transpl, 2008; 14(12); 1718-24
24. Klink T, Simon P, Knopp C, Liver remnant regeneration in donors after living donor liver transplantation: Long-term follow-up using CT and MR imaging: Rofo, 2014; 186(6); 598-605
25. Fausto N, Campbell JS, Riehle KJ, Liver regeneration: J Hepatol, 2012; 57(3); 692-94
26. Fausto N, Campbell JS, Riehle KJ, Liver regeneration: Hepatology, 2006; 43(2 Suppl 1); S45-S53
27. Humar A, Kosari K, Sielaff TD, Liver regeneration after adult living donor and deceased donor split-liver transplants: Liver Transpl, 2004; 10(3); 374-78
28. Park J, Kim JH, Kim JE, Prediction of liver regeneration in recipients after living-donor liver transplantation in using preoperative CT texture analysis and clinical features: Abdom Radiol (NY), 2020; 45(11); 3763-74
29. Olthoff KM, Emond JC, Shearon TH, Liver regeneration after living donor transplantation: Adult-to-adult living donor liver transplantation cohort study: Liver Transpl, 2015; 21(1); 79-88
30. Yamamoto KN, Ishii M, Inoue Y, Prediction of postoperative liver regeneration from clinical information using a data-led mathematical model: Sci Rep, 2016; 6; 34214
31. Groeneveld D, Pereyra D, Veldhuis Z, Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans: Blood, 2019; 133(11); 1245-56
32. Kirschbaum M, Jenne CN, Veldhuis ZJ, Transient von Willebrand factor-mediated platelet influx stimulates liver regeneration after partial hepatectomy in mice: Liver Int, 2017; 37(11); 1731-37
33. Tatsumi K, Ohashi K, Taminishi S, Regulation of coagulation factors during liver regeneration in mice: Mechanism of factor VIII elevation in plasma: Thromb Res, 2011; 128(1); 54-61
34. Lesurtel M, Graf R, Aleil B, Platelet-derived serotonin mediates liver regeneration: Science, 2006; 312(5770); 104-7
35. Matsuo R, Nakano Y, Ohkohchi N, Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy: Ann Surg, 2011; 253(4); 759-63
36. Han S, Park HW, Song JH, Association between intraoperative platelet transfusion and early graft regeneration in living donor liver transplantation: Ann Surg, 2016; 264(6); 1065-72
37. Lisman T, Porte RJ, Mechanisms of platelet-mediated liver regeneration: Blood, 2016; 128(5); 625-29
38. Matsuo R, Ohkohchi N, Murata S, Platelets strongly induce hepatocyte proliferation with IGF-1 and HGF in vitro: J Surg Res, 2008; 145(2); 279-86
39. Kurokawa T, Ohkohchi N, Platelets in liver disease, cancer and regeneration: World J Gastroenterol, 2017; 23(18); 3228-39
40. Takahashi K, Liang C, Oda T, Ohkohchi N, Platelet and liver regeneration after liver surgery: Surg Today, 2020; 50(9); 974-83
41. Preziosi ME, Monga SP, Update on the mechanisms of liver regeneration: Semin Liver Dis, 2017; 37(2); 141-51
42. Li N, Hua J, Immune cells in liver regeneration: Oncotarget, 2017; 8(2); 3628-39
Figures
 Figure 1. Flow chart of patient enrolment. (Created with PowerPoint 2016, Microsoft Corporation).
Figure 1. Flow chart of patient enrolment. (Created with PowerPoint 2016, Microsoft Corporation). Figure 2. Liver segmentation using Mimics software program. (A, B), the liver was recognized in CT images. The green lines surround the scope of liver reconstruction. The purple and blue lines surround the hepatic veins and portal veins and their intrahepatic branches, respectively. (C, D), a whole liver was separated into left lobe (purple) and right lobe (light green), and the portal veins (blue), hepatic arteries (red) and biliary ducts (green) were reconstructed. (Created with Mimics 19.0 software)
Figure 2. Liver segmentation using Mimics software program. (A, B), the liver was recognized in CT images. The green lines surround the scope of liver reconstruction. The purple and blue lines surround the hepatic veins and portal veins and their intrahepatic branches, respectively. (C, D), a whole liver was separated into left lobe (purple) and right lobe (light green), and the portal veins (blue), hepatic arteries (red) and biliary ducts (green) were reconstructed. (Created with Mimics 19.0 software) Figure 3. Serial changes in the serum inflammatory biomarker levels in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (A) White blood cell (WBC) count, (B) Neutrophils, (C) Leukocytes. (Created with R version 3.6.2)
Figure 3. Serial changes in the serum inflammatory biomarker levels in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (A) White blood cell (WBC) count, (B) Neutrophils, (C) Leukocytes. (Created with R version 3.6.2) Figure 4. Serial changes in (A) platelets (PLT), (B) prothrombin time (PT), and (C) activated partial thromboplastin time (APTT) in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (Created with R version 3.6.2)
Figure 4. Serial changes in (A) platelets (PLT), (B) prothrombin time (PT), and (C) activated partial thromboplastin time (APTT) in the low- and high-regeneration index (RI) groups on Days 1, 3, and 7 after liver transplantation. (Created with R version 3.6.2) Figure 5. The area under the receiver operating characteristic curve (AUROC) for the combination of the biomarkers of white blood cell (WBC) count, neutrophils, leukocytes, platelets, prothrombin time (PT), and activated partial thromboplastin time APTT on Days 1, 3, and 7 after transplantation. (Created with SPSS version 25.0, IBM Corporation).
Figure 5. The area under the receiver operating characteristic curve (AUROC) for the combination of the biomarkers of white blood cell (WBC) count, neutrophils, leukocytes, platelets, prothrombin time (PT), and activated partial thromboplastin time APTT on Days 1, 3, and 7 after transplantation. (Created with SPSS version 25.0, IBM Corporation). Tables
 Table 1. Characteristics of donors and recipients.
Table 1. Characteristics of donors and recipients. Table 2. Comparisons of characteristics of grafts and clinical parameters of recipients between low-RI and high-RI groups.
Table 2. Comparisons of characteristics of grafts and clinical parameters of recipients between low-RI and high-RI groups. Table 3. Comparisons of postoperative laboratory parameters between high-RI and low-RI groups.
Table 3. Comparisons of postoperative laboratory parameters between high-RI and low-RI groups. Table 4. Association of parameters between high-RI and low-RI groups in the GEE analysis.
Table 4. Association of parameters between high-RI and low-RI groups in the GEE analysis. Table 5. AUROC for combination of WBC, neutrophil, lymphocyte, platelet, PT, and APTT on Days 1, 3, and 7.
Table 5. AUROC for combination of WBC, neutrophil, lymphocyte, platelet, PT, and APTT on Days 1, 3, and 7. Table 1. Characteristics of donors and recipients.
Table 1. Characteristics of donors and recipients. Table 2. Comparisons of characteristics of grafts and clinical parameters of recipients between low-RI and high-RI groups.
Table 2. Comparisons of characteristics of grafts and clinical parameters of recipients between low-RI and high-RI groups. Table 3. Comparisons of postoperative laboratory parameters between high-RI and low-RI groups.
Table 3. Comparisons of postoperative laboratory parameters between high-RI and low-RI groups. Table 4. Association of parameters between high-RI and low-RI groups in the GEE analysis.
Table 4. Association of parameters between high-RI and low-RI groups in the GEE analysis. Table 5. AUROC for combination of WBC, neutrophil, lymphocyte, platelet, PT, and APTT on Days 1, 3, and 7.
Table 5. AUROC for combination of WBC, neutrophil, lymphocyte, platelet, PT, and APTT on Days 1, 3, and 7. In Press
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
02 Apr 2024 : Original article
Liver Transplantation from Brain-Dead Donors with Hepatitis B or C in South Korea: A 2014-2020 Korean Organ...Ann Transplant In Press; DOI: 10.12659/AOT.943588
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








