Abstract
Background
Pancreatic ductal adenocarcinomas (PDACs) are sometimes diagnosed accompanied by rapidly impaired diabetes (PDAC-RID). Although this type of PDAC may have unusual biological features, these features have not been explained.
Methods
Patients with PDAC who underwent upfront pancreatectomy between 2010 and 2018 were retrospectively reviewed. PDAC-RID was defined as a glycated hemoglobin (HbA1c) value of ≥ 8.0% of newly diagnosed diabetes, and acute exacerbation of previously diagnosed diabetes. Other patients were classified as PDAC with stable glycometabolism (PDAC-SG). Clinicopathological factors, long-term survival rates, and recurrence patterns were evaluated.
Results
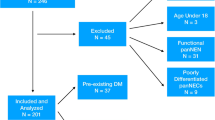
Of the 520 enrolled patients, 104 were classified as PDAC-RID and 416 as PDAC-SG. There was no significant difference regarding TNM staging, resectability, or adjuvant chemotherapy rate between the groups. However, 5-years cancer-specific survival (CSS) was significantly higher in the PDAC-RID group than in the PDAC-SG group (45.3% vs. 31.1%; p = 0.02). This survival difference was highlighted in relatively early-stage PDAC (≤ pT2N1) (CSS: 60.8% vs. 43.6%; p = 0.01), but the difference was not significant for advanced-stage PDAC. A multivariate analysis of early-stage PDAC showed that PDAC-SG was an independent risk factor of shorter CSS (hazard ratio 1.76; p = 0.02). The hematogenous metastatic rate in early-stage PDAC was lower in the PDAC-RID group than in the PDAC-SG group (18.3% vs. 35.8%; p = 0.01).
Conclusions
PDAC-RID showed a favorable long-term survival rate after curative resection with low hematogenous metastases, which may be due to its unique biology.



Similar content being viewed by others
REFERENCES
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N England J Med. 2014;371(11):1039–49.
Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326(9):851–62.
Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–22.
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–57.
Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10(7):423–33.
Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. 2019;156(7):2024–40.
Roy A, Sahoo J, Kamalanathan S, Naik D, Mohan P, Kalayarasan R. Diabetes and pancreatic cancer: exploring the two-way traffic. World J Gastroenterol. 2021;27(30):4939–62.
Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1(3):226–37.
Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):981–7.
Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology. 2012;143(6):1510-7.e1.
Kiritani S, Ono Y, Takamatsu M, Oba A, Sato T, Ito H, et al. Diabetogenic liver metastasis from pancreatic cancer: a case report. Surg Case Rep. 2022;8(1):224.
Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes care. 2022;45 Suppl 1:S17–s38.
Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Comprehens Cancer Netw. 2017;15(8):1028–61.
Inoue Y, Saiura A, Oba A, Kawakatsu S, Ono Y, Sato T, et al. Optimal extent of superior mesenteric artery dissection during pancreaticoduodenectomy for pancreatic cancer: balancing surgical and oncological safety. J Gastrointestin Surg. 2019;23(7):1373–83.
14. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2018. Diabetes care. 2018;41 Suppl 1:S144-s51.
Inoue Y, Saiura A, Yoshioka R, Ono Y, Takahashi M, Arita J, et al. Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach. Ann Surg. 2015;262(6):1092–101.
Sato T, Inoue Y, Takahashi Y, Mise Y, Ishizawa T, Tanakura K, et al. Distal pancreatectomy with celiac axis resection combined with reconstruction of the left gastric artery. J Gastrointestin Surg. 2017;21(5):910–7.
Inoue Y, Saiura A, Takahashi Y. A novel classification and staged approach for dissection along the celiac and hepatic artery during pancreaticoduodenectomy. World J Surg. 2018;42(9):2963–7.
Oba A, Ito H, Ono Y, Sato T, Mise Y, Inoue Y, et al. Regional pancreatoduodenectomy versus standard pancreatoduodenectomy with portal vein resection for pancreatic ductal adenocarcinoma with portal vein invasion. BJS Open. 2020;4(3):438–48.
Brierley JD, Gospodarowicz MK, Wittekind C. International union against cancer (UICC). TNM classification of malignant tumors. 8th edition. Chichester: Wiley-Blackwell; 2017.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584–91.
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–8.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134(1):95–101.
Yuan C, Babic A, Khalaf N, Nowak JA, Brais LK, Rubinson DA, et al. Diabetes, weight change, and pancreatic cancer risk. JAMA Oncol. 2020;6(10):e202948.
Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. J Diab Investig. 2020;11(4):1020–76.
Lv X, Qiao W, Leng Y, Wu L, Zhou Y. Impact of diabetes mellitus on clinical outcomes of pancreatic cancer after surgical resection: a systematic review and meta-analysis. PloS One. 2017;12(2):e0171370.
Chu CK, Mazo AE, Goodman M, Egnatashvili V, Sarmiento JM, Staley CA, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17(2):502–13.
Dandona M, Linehan D, Hawkins W, Strasberg S, Gao F, Wang-Gillam A. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas. 2011;40(6):931–7.
Balzano G, Dugnani E, Gandolfi A, Scavini M, Pasquale V, Aleotti F, et al. Effect of diabetes on survival after resection of pancreatic adenocarcinoma: a prospective, observational study. PloS One. 2016;11(11):e0166008.
Walter U, Kohlert T, Rahbari NN, Weitz J, Welsch T. Impact of preoperative diabetes on long-term survival after curative resection of pancreatic adenocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21(4):1082–9.
Benhamou PY, Vuillez JP, Halimi S, Meffre G, Bachelot I. Influence of metabolic disturbances of diabetes mellitus on serum CA 19–9 tumor marker. Diabete Metab. 1991;17(1):39–43.
Evan T, Wang VM, Behrens A. The roles of intratumour heterogeneity in the biology and treatment of pancreatic ductal adenocarcinoma. Oncogene. 2022;41(42):4686–95.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34.
Japan Pancreas Society. General Rules for the Study of Pancreatic Cancer, 7th edition revised and enlarged version. Kanehara & Co., Ltd; 2020.
Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4(7):963–9.
Hank T, Sandini M, Qadan M, Weniger M, Ciprani D, Li A, et al. Diabetes mellitus is associated with unfavorable pathologic features, increased postoperative mortality, and worse long-term survival in resected pancreatic cancer. Pancreatology. 2020;20(1):125–31.
Ma J, Wang J, Ge L, Long B, Zhang J. The impact of diabetes mellitus on clinical outcomes following chemotherapy for the patients with pancreatic cancer: a meta-analysis. Acta Diabetol. 2019;56(10):1103–11.
Rajamanickam ES, Christians KK, Aldakkak M, Krepline AN, Ritch PS, George B, et al. Poor glycemic control is associated with failure to complete neoadjuvant therapy and surgery in patients with localized pancreatic cancer. J Gastrointestin Surg. 2017;21(3):496–505.
Acknowledgment
This research was supported by a Grant-in-Aid for Research from the National Center for Global Health and Medicine (19A2013). The authors thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
Sho Kiritani, Yoshihiro Ono, Manabu Takamatsu, Sachiyo Yoshio, Mamiko Miyashita, Atsushi Oba, Takafumi Sato, Hiromichi Ito, Yosuke Inoue, Akio Saiura, and Yu Takahashi have no conflicts of interest associated with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2023_14408_MOESM1_ESM.tif
Supplementary fig.1 1 Subgroup analysis of CSS according to T and N factors. (a–c) Kaplan–Meier curves based on the T factor. (d–f) Kaplan–Meier curves based on the N factor. CSS cancer-specific survival, PDAC-RID pancreatic ductal adenocarcinoma accompanied by rapidly impaired diabetes, PDAC-SG pancreatic ductal adenocarcinoma with stable glycometabolism (TIF 87 KB)
10434_2023_14408_MOESM7_ESM.tif
Supplementary fig. 2 Distribution of subdivided venous invasion between PDAC-RID and PDAC-SG. PDAC-RID pancreatic ductal adenocarcinoma accompanied by rapidly impaired diabetes, PDAC-SG pancreatic ductal adenocarcinoma with stable glycometabolism, v0 no evidence of venous invasion, v1 slight venous invasion, v2 moderate venous invasion, v3 marked venous invasion (TIF 91 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kiritani, S., Ono, Y., Takamatsu, M. et al. Unique Biology of Pancreatic Ductal Adenocarcinoma Accompanied by Rapidly Impaired Diabetes: A Favorable Long-Term Survival Following Curative Resection. Ann Surg Oncol 31, 514–524 (2024). https://doi.org/10.1245/s10434-023-14408-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14408-0