Abstract
Objective
The aim of this study was to compare the short- and long-term outcomes of laparoscopic surgery (LS) and open surgery (OP) for perihilar cholangiocarcinoma (PHC) using a large real-world dataset in China.
Methods
Data of patients with PHC who underwent LS and OP from January 2013 to October 2018, across 10 centers in China, were extracted from medical records. A comparative analysis was performed before and after propensity score matching (PSM) in the LS and OP groups and within the study subgroups. The Cox proportional hazards mixed-effects model was applied to estimate the risk factors for mortality, with center and year of operation as random effects.
Results
A total of 467 patients with PHC were included, of whom 161 underwent LS and 306 underwent OP. Postoperative morbidity, such as hemorrhage, biliary fistula, abdominal abscess, and hepatic insufficiency, was similar between the LS and OP groups. The median overall survival (OS) was longer in the LS group than in the OP group (NA vs. 22 months; hazard ratio [HR] 1.19, 95% confidence interval [CI] 1.02–1.39, p = 0.024). Among the matched datasets, OS was comparable between the LS and OP groups (NA vs. 35 months; HR 0.99, 95% CI 0.77–1.26, p = 0.915). The mixed-effect model identified that the surgical method was not associated with long-term outcomes and that LS and OP provided similar oncological outcomes.
Conclusions
Considering the comparable long-term prognosis and short-term outcomes of LS and OP, LS could be a technically feasible surgical method for PHC patients with all Bismuth–Corlett types of PHC.
Similar content being viewed by others
Perihilar cholangiocarcinoma (PHC) is one of the most dismal malignancies involving the confluence of the hepatic ducts, and contributes to >50% of malignant tumors of the biliary tract.1,2 Owing to its insensitivity to radiotherapy and chemotherapy, surgical resection has become a potentially curative therapeutic option for PHC.3 However, due to its aggressiveness, late presentation, and refractory nature, most patients are admitted following a late clinical diagnosis, with macrovascular invasion, lymph node or liver parenchyma involvement, resulting in only 10–15% of patients being eligible for resection with curative intent.4,5 Even after resection, the 5-year survival rate remains disappointing at around 10–35%.6,7,8
The surgical difficulty for PHC is known as the Mount Everest of Abdominal Surgery.9 Conventional radical resection of PHC includes complete resection of the extrahepatic bile duct, extended hemihepatectomy with complete caudate lobectomy, lymph node dissection in the hepatoduodenal ligament, and choledochojejunostomy.10,11 With improvements in laparoscopic techniques and the gradual establishment of laparoscopic surgical procedures, advancements in laparoscopic resection have revolutionized the process for most abdominal surgeries, including resection for colorectal cancer,12,13,14 pancreatoduodenectomy,15,16 all types of hepatobiliary resections,17,18,19,20 and cholangiocarcinoma surgery.21 Laparoscopic surgery (LS) for PHC has been shown to be safe and feasible, with comparable short-term outcomes as open surgery (OP).22,23 However, most LSs for PHC are limited to carefully selected patients and are technically achievable for experienced surgeons. High-volume and comparative studies are lacking, and the evidence is undoubtedly biased.24 Moreover, the long-term outcome of LS for PHC is lacking owing to the limited laparoscopic experience and the absence of long-term follow-up as this is a newly applied technique.
It is imperative to undertake large-scale multicenter analyses to investigate the technical feasibility and safety of LS for PHC. Therefore, we performed a multicenter real-world study to compare the long-term survival of PHC patients who underwent LS or OP, to summarize the updated applications, advancements, and limitations of LS for treating PHC and help with decision making during treatment.
Materials and Methods
Patient Selection
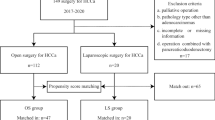
A retrospective review of real-world institutional databases from 10 hospitals in China identified 467 patients with PHC who underwent curative surgery (including R0 and R1) from January 2013 to October 2018. Patients with pathologically confirmed PHC and no evidence of distant metastasis on preoperative examination were included. Those who underwent combined hepato-pancreaticoduodenal resection, extrahepatic bile duct resection only, were lost to follow-up, or had missing data on the main outcomes, were excluded. All included cases met the resectability criteria laid down by the National Comprehensive Cancer Network guidelines for preoperative assessments.25 All cases were systematically discussed during multidisciplinary hepatobiliary meetings, involving experienced surgeons, radiologists, endoscopists, oncologists, radiation specialists, and pathologists, to define the indications and characteristics of the surgical procedure and to share the steps of perioperative optimization. This study was approved by the Ethics Committee of Tongji Hospital (approval number TJ-IRB20220531). This work has been reported in line with the STROCSS (Strengthening the Reporting of Cohort Studies in Surgery) criteria.26 All participating centers were high-volume hepatic surgical centers, and the surgical teams were experienced in both LS and OP.
Surgical Technique and Follow-Up
Preoperatively, all patients underwent three-dimensional visualization to clearly show the intrahepatic pipeline, size and location of tumors, and the relationship between the tumor and intrahepatic pipeline. LS was defined as a total laparoscopic surgery. Surgical procedures included hepatectomy with en bloc resection of the caudate lobe and extrahepatic bile duct as well as regional lymph node dissection. The procedure for PHC included (1) local excision of hilar bile ducts only; (2) left hemihepatectomy; (3) right hemihepatectomy; (4) extended left hemihepatectomy; (5) extended right hemihepatectomy; and (6) segmentectomy (≤3 Couinaud segments). Frozen section examination of the proximal and distal bile duct resection margins was routinely performed intraoperatively. The surgical techniques, steps, and principles of surgery are similar between the laparoscopic and open approaches.
Data Collection and Definitions
Baseline characteristics included patient age, sex, body mass index, American Society of Anesthesiologists score,27 year of operation, tumor differentiation, M stage, T stage, N stage, TNM stage, and history of adjuvant treatment. The TNM staging was based on the American Joint Committee on Cancer (AJCC) staging system (8th edition).28 The primary endpoint was long-term overall survival (OS) after initial radical surgery, which was defined as the duration from the first postoperative day to either the date of death or last follow-up. Secondary endpoints were perioperative outcomes, including postoperative complications, reoperation, mortality within 30 and 90 days, readmission within 90 days, and postoperative length of stay (LOS). Postoperative complications were reviewed within 90 days after surgery and were graded according to the Clavien–Dindo (CD) classification system.29 Postoperative biliary leakage,30 hemorrhage,31 and liver failure32 were defined and classified according to the criteria established by the International Study Group of Liver Surgery (ISGLS). Wound infection was defined as purulent drainage from the incision and/or positive culture findings of the fluid or tissue aseptically obtained from the incision. Operative details, including operation duration, intraoperative blood loss (IBL), blood transfusion, vascular resection, number of resected lymph nodes, and R0 resection, were also analyzed. R0 resection was defined as tumor-free margins in all reported surgical margins (biliary and circumferential). The definitions of all these parameters were unified by all participating teams at the beginning of this study. All patients were recommended to return for follow-up at the outpatient department 1 month after discharge, every 3–6 months for the first 2 years, and annually thereafter. Survival data were collected by searching the electronic outpatient system or by telephone interviews. Final follow-up was conducted in January 2020.
Statistical Analyses
To minimize the potential bias from confounding factors between the OP and LS groups in real-world data, propensity matching was performed to create a pseudo-randomized population. LS and OP were matched 1:1 using the nearest-neighbor matching method without replacement. A caliper radius equal to a standard deviation (SD) of 0.1 was set to prevent poor matching.
Continuous variables were expressed as medians and interquartile ranges (IQRs) or mean (SD), while categorical variables were expressed as numbers (n) and percentages (%). Independent-samples t-tests were performed to compare continuous variables that followed normal distributions; otherwise, the Mann–Whitney U test was used. Chi-square or Fisher’s exact tests were used to compare categorical variables. Survival analyses were conducted using the Kaplan–Meier method with log-rank tests. Multivariate Cox regression analyses were used to estimate the risk factors for long-term all-cause mortality. To further consider the measures of hidden confounders, the period of operation (2013–2018) and the centers (e.g., improved perioperative care in recent years and different surgical management in different hospitals) were included as random effects in the mixed-effects Cox regression model. The results are presented as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). The proportional hazards assumption of the Cox proportional regression model was assessed by eyeballing the Kaplan–Meier plot and the log-minus-log plot.33 All statistical procedures were conducted using SAS software version 9.40 (SAS Institute, Inc., Cary, NC, USA). Two-sided hypothesis testing with a predetermined level of p < 0.05 was considered statistically significant.
Results
Patient Clinicopathological Characteristics and Pathologic Features
Among the 467 PHC patients, 161 underwent LS and 306 underwent OP (Fig. 1). The percentage of LS in these hospitals increased from 36.36% (12 of 33) in 2013 to 38.24% (26 of 48) in 2018. The median follow-up period was 24 months (IQR 13–75) for the entire cohort, 21 months (IQR 13–32) for the LS cohort, and 28 months (IQR 14–42) for the OP cohort. In the original cohort, preoperative liver function indices, such as total bilirubin (Tbil), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), were significantly lower in the LS group. A higher TNM stage (TVa and TVb) was observed in the OP group (p < 0.001). There was no statistical difference in the Bismuth–Corlett types between LS and OP, with the following details: I in 16 (3.4%), IIA in 108 (23.1%), IIB in 102 (21.8%), IIIA in 44 (9.4%), IIIB in 61 (13.1%), and IV in 134 (26.7%) patients. After propensity score matching (PSM), 83 patients in the OP group were well matched with 83 patients in the LS group and comprised the matched cohort. The baseline data are shown in Table 1.
Operative Characteristics and Related Short-Term Outcomes
The surgical characteristics of the patients and the related short-term outcomes in the LS and OP groups are shown in Table 2. In the original cohort, patients who underwent LS had comparable operative time (median, 350 vs. 345 min, p = 0.054), blood loss (median, 250 vs. 300 mL, p = 0.859), and R0 resection (91.3% vs. 91.8%, p = 0.845) with OP. However, more enlarged hepatectomy, less vascular resection, and less biliary plasty were observed in the LS group. Furthermore, a higher percentage of people received postoperative chemotherapy in the LS group compared with the OP group (44.72% vs. 15.36%, p < 0.0001). In the matched cohort, except for less vascular resection observed in the LS group, the other operative characteristics were comparable between the two groups.
The median postoperative LOS was shorter (13 vs. 15 days, p = 0.0006) and the overall postoperative morbidity rate was significantly lower in the LS group than in the OP group (31.1% vs. 41.5%, p = 0.027), with severe complications (CD ≥III) accounting for 13.0% in the LS group compared with 24.8% in the OP group (p = 0.003). Major postoperative complications such as biliary fistula and hemorrhage were comparable between the two groups. After matching, the complication and reoperation rates were similar between the two groups. Overall, the LS group was associated with significant LOS reduction (median, 14 vs. 15 days, p = 0.022) and a shorter postoperative drainage tube keep (PDTK) time (median, 6 vs. 8 days, p = 0.035) than the OP group.
Long-Term Outcomes and Overall Survival
By October 2018, 212 patients (45.4%) had died. The 90-day mortality rate was 7.45% (12 of 161) in the LS cohort and 9.48% (29 of 306) in the OP cohort. These 41 patients were excluded from the long-term mortality analysis. The 1-year (72.2% vs. 64.6%) and 2-year (57.9% vs. 48.0%) OS rates in the LS group were higher than those in the OP group (p < 0.022) before matching. The median OS was NA months in the LS group and 22 months in the OP group (HR 1.19, 95% CI 1.02–1.39, p = 0.024) (Fig. 2a). After matching, the year-specific survival rates were similar between the two groups (1-year [71.3% vs. 70.2%] and 2-year [52.1% vs. 48.4%], with similar mortality observed in the LS and OP groups [log-rank test, p = 0.912]). The median OS was NA and 35 months in the LS and OP groups, respectively (HR 0.99, 95% CI 0.77–1.26, p = 0.915) [Fig. 2b]. In addition, the OS of the two surgical groups was also stratified by subgroups, and the results showed that females and patients older than 60 years of age can benefit from LS surgery (Fig. 3a). When patients were well-matched, the surgical outcomes did not differ between the two surgical groups (Fig. 3b). The year-specific survival rates for most subgroup characteristics are shown in electronic supplementary Table S1.
Forest plot of risk evaluation of LS compared with OP for PHC patients in different subgroups. a Before matching; b after matching. PHC perihilar cholangiocarcinoma, LS laparoscopic surgery, OP open surgery, IQR interquartile range, HR hazard ratio, CI confidence interval, BMI body mass index, AJCC American Joint Committee on Cancer
Table 3 shows the results of univariate and multivariate Cox proportional hazards mixed-effects models for OS, with center and year of operation as the random effects. The proportional hazard assumption was not violated (p = 0.224). The estimated coefficients for each predictor are shown in Fig. 4. Higher AST levels, Bismuth–Corlett type, CA19-9 levels, age >65 years, and higher AJCC stage were key risk predictors of long-term survival (HR 1.7 to +4.8). Being female, receiving a stent, and receiving chemotherapy were key protective predictors (HR 0.4 to +0.6). Compared with these fixed effects, the between-center variation (HR 0.5 to +2.1) and between-year variation (HR 0.8 to +1.1) were much smaller than the fixed-effects predictors (electronic supplementary Fig. S1).
Fixed-effects estimate for OS predictors. The strongest patient-factor predictors are higher CA19-9 level, higher AJCC stage, older age, higher Bismuth–Corlett type, had postoperative complication, and higher level of AST. OS overall survival, AJCC American Joint Committee on Cancer, AST aspartate aminotransferase, BMI body mass index, ASA American Society of Anesthesiologists
Postoperative Outcomes According to Bismuth Type
In the present study, 226 patients with PHC had a low Bismuth type (Bismuth I/II) and 239 patients had a high Bismuth type (Bismuth III/IV), with significantly different surgical characteristics and OS. OS was much better in the low Bismuth type (median OS, NA; 95% CI 15–NA months) than in the high Bismuth type (median OS, 15; 95% CI 7–40 months, p < 0.0001) [electronic supplementary Table S2 and Fig. S2]. Furthermore, shorter LOS and less IBL were observed in patients who underwent LS compared with those who underwent OP among patients with Bismuth types I–II, with other characteristics being comparable between the groups. The OS was longer in the LS group than in the OP group, and the median OS was NA (IQR 20–NA) and 41 (95% CI 14–NA) months in both groups (p = 0.0469). Among patients with Bismuth types III–IV, those who underwent LS showed comparable or better short-term outcomes than the OP group, such as similar postoperative complications, shorter LOS (median, 14 vs. 17 days), and lower rates of severe complications (12.66% vs. 27.5%), demonstrating guaranteed safety of laparoscopic resection for high Bismuth types of PHC. However, a longer operation time, less vascular resection and biliary plasty, and higher postoperative hemorrhage were observed in the LS group. The OS was comparable between the LS and OP groups, with a median OS of 15 (IQR 8–NA) and 14 (95% CI 6–40) months in both groups (p = 0.4285) [electronic supplementary Fig. S3].
Discussion
LS remains technically challenging and is usually performed in selected patients in a few high-volume centers. In the present study, we enrolled a large sample of patients with PHC who underwent LS, and compared the safety and long-term efficacy of LS with OP. Similar OS was observed between the two surgical groups in the well-matched cohort. However, a longer OS was observed in the LS group in the original cohort. The cause of such variation may be related to patient selection before surgery, which can be reflected by the baseline differences between the LS and OP groups, particularly significantly more females and better liver function in the LS group, and the demonstrated independent protective factors for OS in the original cohort. Assuming no selection bias, LS had similar postoperative short-term safety and long-term survival compared with OP, with evidence from multicentric practice. This also suggests that long-term survival can be achieved in some PHC patients, regardless of the surgical method.
Laparoscopic techniques were first used to determine the PHC staging and assess whether the tumor could be surgically resected.34 Recently, with the improvement of laparoscopic technology and instruments, combined with the accumulation of surgical experience and the improvement of surgical skills, the application of LS in PHC has been gradually developed and has shown promising short-term outcomes.35,36 Laparoscopy has the effect of magnification and blind area traversability, and thus has several advantages due to the clear visual field: the first, second, and third porta hepatis are more clearly exposed; convenient, highly selective separation of blood vessels entering and exiting the liver; and identification of the variant hepatic artery, portal vein, and bile duct. Theoretically, LS can provide satisfactory safety during complex surgical procedures. However, the complex location of PHC, adjacent to the hepatic artery, portal vein, and hepatic parenchyma, and the complex surgical resections for PHC, including liver resection, bilio-enteric reconstruction, radical lymph node dissection around the perihilar, retropancreatic, and para-aortic areas, as well as the high incidence of postoperative complications, have hindered the development of LS in PHC, which has been far behind other abdominal surgeries. In the present study, LS showed a similar R0 resection rate, lymph node retrieval, and operative time as OP. The LOS was shorter and the incidence of severe postoperative complications (CD >III) was lower in the LS group. All the evidence presented the acceptability of LS in PHC applications to obtain optimized short-term safety.
Laparoscopy in PHC evolved rapidly during the study period (from 2013 to 2018). Most notably, the number of PHC patients with stage III/IV LS increased annually during the study period from 25 to 60%. The attention of surgical improvement towards PHC has focused on the technical methods of controlling microscopic spread of disease to achieve long-term survival and optimize short-term surgical results.37,38 Most previous studies have guaranteed short-term outcomes. However, the paucity of reports in the literature focusing on long-term evidence suggests that a step towards the systematization of the laparoscopic approach has not yet been taken due to a conceptual barrier. A recent analysis of patients with PHC treated in one of the leading European centers for LS showed no oncologic inferiority of laparoscopic resection, which posed a major concern to surgeons preparing for the final step toward minimally invasive PHC surgery.39 In the present study, the median OS in the LS group was higher than that in the OP group, suggesting better survival following LS for some well-selected patients.
The complexity of surgical procedures for PHC and survival is mainly dependent on the Bismuth–Corlett type. Laparoscopy has been used for all Bismuth types, although it is predominantly used in patients with low-stage PHCs.40,41,42 For PHC patients with high Bismuth type (III/IV), surgery involves caudate lobectomy, complex biliojejunal anastomosis, multiple biliary tract reconstruction, and vascular reconstruction, which dramatically increases the complexity of the operation.43 Laparoscopic caudate lobe resection is a feasible and safe procedure.44,45 The three-dimensional visualization technique can be used to accurately evaluate the scope of tumor status as well as the invasion status of peripheral blood vessels and bile ducts. Therefore, it is possible to develop a detailed surgical plan using multidisciplinary instructions for complex surgery.46 In addition, through external liver suspension during the operation, the hilum can be better exposed, and the operation field can significantly expand.47 To date, several attempts have been made to perform laparoscopic resection of the Bismuth III/IV type, and some promising short-term clinical outcomes have been achieved. In the present study, LS showed comparable or better short-term outcomes in PHC with Bismuth III/IV type compared with OP, such as similar postoperative complications, shorter LOS, and lower severity of complications, indicating the guaranteed safety of laparoscopic resection for PHC with high Bismuth classification. However, the longer operation time in LS than in OP indicates that the LS for PHC is still in its learning stage.
Postoperative adjuvant radiotherapy and chemotherapy have become the major treatment options for malignant tumors. However, the role of adjuvant therapy and the exact postoperative regimens for PHC remain controversial, with no prospective randomized controlled studies on this issue at present. In the 2019 American Society of Clinical Oncology (ASCO) clinical practice guidelines, oral capecitabine was recommended as adjuvant chemotherapy following surgery for patients with resected biliary tract cancer, based on the results of the BILCAP randomized controlled trial; however, subgroup analysis of patients with PHC failed to yield positive results.48 Some retrospective reports have suggested that adjuvant treatment could improve the OS of patients with PHC with lymph node metastases49,50 or with positive resection margins.51 Based on the ASCO clinical practice guidelines, adjuvant chemoradiotherapy is recommended for patients with PHC who undergo R1 resection.52 The present study suggests that adjuvant chemotherapy is a significant protective prognostic factor for long-term survival in patients with PHC. Subgroup analyses of Bismuth type III–IV patients with R0 resection revealed that adjuvant chemotherapy produced significant survival benefits when compared with surgery alone (electronic supplementary Fig. S4); a similar result was reported by Im et al.53 However, a relatively low percentage of patients received adjuvant chemotherapy and there was a large difference in chemotherapy percentages between the LS and OP groups. A previous study demonstrated that LS is associated with greater rates of compliance with guidelines for adjuvant chemotherapy, as well as a slightly shorter time before initiation of chemotherapy.54 Faster postoperative recovery and decreased postoperative complications could increase the likelihood of receiving adjuvant chemotherapy after LS compared with OP. With the cumulative evidence supporting the necessity for adjuvant therapy, improving compliance to guidelines for adjuvant chemotherapy and reaching a consensus regarding the detailed implementation plan for adjuvant treatment needs to be further validated through strict postoperative patient management in future studies. Other risk factors such as elevated CA19-9 level reflecting higher tumor burden,55,56 elevated AST level reflecting poor liver status,57 and older age reflecting poor physical function, were all associated with poor survival outcomes. Surgical resection with negative histologic margins (R0 resection) was considered the only option for long-term survival in patients with PHC.58,59 The R0 rates in the LS and OP groups were 91.3% and 91.83%, respectively. The high negative resection rate in this analysis guarantees postoperative benefit for patients with PHC in both the LS and OP groups.
The results for the overall experience were positive; however, the methodological and technical limitations of the present study may be due to its retrospective nature and small sample size. The LS and OP cases were obtained from 10 institutions, and potential selection bias could not be avoided. In addition, the relatively short follow-up period, especially in the LS group, limited sufficient analyses of long-term survival between the LS and OP groups. Additionally, oncological outcomes, such as recurrence-free survival or disease-free survival, were not documented; therefore, we could not thoroughly compare the disease progression process between the two surgical groups. Finally, with the increasing popularity of robotic platforms for complex hepatic resections and reconstructions worldwide,60 we did not include the robotic surgeries because of the relatively small number of cases during the research period. In future studies, we will include more patients who underwent different types of PHC surgeries to further investigate individualized surgical treatment strategies.
Conclusions
LS could be technically feasible and achieve equivalent long-term survival as OP in patients with resectable PHC, regardless of the Bismuth–Corlett type. Additionally, LS can shorten the length of hospital stay and reduce the occurrence of severe postoperative complications. However, considering the steep learning curve and high risks involved, this procedure should be performed by experienced surgeons after adequate training in high-volume laparoscopic liver centers.
References
Nath MC, Torbenson MS, Erickson LA. Perihilar cholangiocarcinoma. Mayo Clinic Proc. 2018;93:397–8.
Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22.
Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593–607.
Lewis HL, Rahnemai-Azar AA, Dillhoff M, et al. Current management of perihilar cholangiocarcinoma and future perspectives. Chirurgia (Bucur). 2017;112:193–207.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–29.
Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg. 2015;221:1041–9.
Kang MJ, Jang JY, Chang J, et al. Actual long-term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg. 2016;40:2451–9.
Ethun CG, Lopez-Aguiar AG, Anderson DJ, et al. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg. 2018;267:797–805.
Hosokawa I, Shimizu H, Yoshidome H, et al. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg. 2014;259:1178–85.
Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–18.
Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17; discussion 17-9.
Park JW, Kang SB, Hao J, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:569–77.
Julien M, Dove J, Quindlen K, et al. Evolution of laparoscopic surgery for colorectal cancer: the impact of the clinical outcomes of surgical therapy group trial. Am Surg. 2016;82:685–91.
Ratti F, Fiorentini G, Cipriani F, et al. Laparoscopic vs open surgery for colorectal liver metastases. JAMA Surg. 2018;153:1028–35.
Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic versus open pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. Annals of surgery. 2020;271(1):54–66.
Wang M, Li D, Chen R, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:438–47.
Görgec B, Benedetti Cacciaguerra A, et al. Assessment of textbook outcome in laparoscopic and open liver surgery. JAMA surgery. 2021;156:e212064.
Wang Y, Ma K, Zhong A, et al. Hepatopulmonary syndrome after radiofrequency ablation of recurrent intrahepatic cholangiocarcinoma: a case report. OncoTargets Ther. 2019;12:2431–8.
de Rooij T, van Hilst J, van Santvoort H, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269:2–9.
Wang M, Peng B, Liu J, et al. Practice patterns and perioperative outcomes of laparoscopic pancreaticoduodenectomy in China: a retrospective multicenter analysis of 1029 patients. Ann Surg. 2021;273(1):145–53.
Jin B, Chen MT, Fei YT, et al. Safety and efficacy for laparoscopic versus open hepatectomy: a meta-analysis. Surg Oncol. 2018;27:a26–34.
Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc. 2016;30:4835–40.
Wang W, Fei Y, Liu J, et al. Laparoscopic surgery and robotic surgery for hilar cholangiocarcinoma: an updated systematic review. ANZ J Surg. 2021;91:42–8.
Hu HJ, Wu ZR, Jin YW, et al. Minimally invasive surgery for hilar cholangiocarcinoma: state of art and future perspectives. ANZ J Surg. 2019;89:476–80.
Nagino M. Surgical treatment of perihilar cholangiocarcinoma: resection or transplant? Ann Surg. 2018;267:806–7.
Agha R, Abdall-Razak A, Crossley E, et al. The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156–65.
Hocevar LA, Fitzgerald BM. American Society of Anesthesiologists Staging. StatPearls Publishing Copyright © 2021. Treasure Island (FL): StatPearls Publishing LLC; 2021.
Gaspersz MP, Buettner S, van Vugt JLA, et al. Evaluation of the New American Joint Committee on Cancer Staging Manual 8th Edition for Perihilar Cholangiocarcinoma. J Gastrointest Surg. 2020;24:1612–8.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–8.
Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 2011;13:528–35.
Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24.
Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–23.
Adam MA, Choudhury K, Dinan MA, et al. Minimally invasive versus open pancreaticoduodenectomy for cancer: practice patterns and short-term outcomes among 7061 patients. Ann Surg. 2015;262:372–7.
Zhang Y, Dou C, Wu W, et al. Total laparoscopic versus open radical resection for hilar cholangiocarcinoma. Surg Endosc. 2020;34:4382–7.
Chen Y, Xu Y, Zhang Y. Current status of laparoscopic radical hilar cholangiocarcinoma in Mainland China. Biosci Trends. 2020;14:168–73.
Ratti F, Cipriani F, Fiorentini G, et al. Management of hilum infiltrating tumors of the liver: the impact of experience and standardization on outcome. Digest Liver Dis. 2019;51:135–41.
Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–40.
Ratti F, Fiorentini G, Cipriani F, et al. Perihilar cholangiocarcinoma: are we ready to step towards minimally invasiveness? Updates Surg. 2020;72:423–33.
Yu H, Wu SD, Chen DX, et al. Laparoscopic resection of Bismuth type I and II hilar cholangiocarcinoma: an audit of 14 cases from two institutions. Digest Surg. 2011;28:44–9.
Zhang CW, Liu J, Hong DF, et al. Pure laparoscopic radical resection for type IIIa hilar cholangiocarcinoma. Surg Endosc. 2018;32:1581–2.
Yu H, Wu SD, Tian Y, et al. Single-incision laparoscopic resection of Bismuth I hilar cholangiocarcinoma. Surg Innov. 2013;20:209–13.
Bhutiani N, Scoggins CR, McMasters KM, et al. The impact of caudate lobe resection on margin status and outcomes in patients with hilar cholangiocarcinoma: a multi-institutional analysis from the US Extrahepatic Biliary Malignancy Consortium. Surgery. 2018;163:726–31.
Abu Hilal M, Badran A, Di Fabio F, et al. Pure laparoscopic en bloc left hemihepatectomy and caudate lobe resection in patients with intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech Part A. 2011;21:845–9.
Levi Sandri GB, Spoletini G, Mascianà G, et al. The role of minimally invasive surgery in the treatment of cholangiocarcinoma. Eur J Surg Oncol. 2017;43:1617–21.
Zhang J, Guo X, Wang H, et al. The application of three-dimensional visualization in preoperative evaluation of portal Vein invasion in hilar cholangiocarcinoma. Cancer Manag Res. 2020;12:9297–302.
Feng F, Cao X, Liu X, et al. Laparoscopic resection for Bismuth type III and IV hilar cholangiocarcinoma: how to improve the radicality without direct palpation. J Surg Oncol. 2019;120:1379–85.
Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–73.
Krasnick BA, Jin LX, Davidson JTt, et al. Adjuvant therapy is associated with improved survival after curative resection for hilar cholangiocarcinoma: a multi-institution analysis from the U.S. extrahepatic biliary malignancy consortium. J Surg Oncol. 2018;117:363–71.
Mizuno T, Ebata T, Yokoyama Y, et al. Adjuvant gemcitabine monotherapy for resectable perihilar cholangiocarcinoma with lymph node involvement: a propensity score matching analysis. Surg Today. 2017;47:182–92.
Nassour I, Mokdad AA, Porembka MR, et al. Adjuvant therapy is associated with improved survival in resected perihilar cholangiocarcinoma: a propensity matched study. Ann Surg Oncol. 2018;25:1193–201.
Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1015–27.
Im JH, Choi GH, Lee WJ, et al. Adjuvant radiotherapy and chemotherapy offer a recurrence and survival benefit in patients with resected perihilar cholangiocarcinoma. J Cancer Res Clin Oncol. 2021;147:2435–45.
Kim RH, Kavanaugh MM, Caldito GC. Laparoscopic colectomy for cancer: Improved compliance with guidelines for chemotherapy and survival. Surgery. 2017;161:1633–41.
Lee JW, Lee JH, Park Y, et al. Prognostic impact of perioperative CA19-9 levels in patients with resected perihilar cholangiocarcinoma. J Clin Med. 2021;10(7):1345.
Chung MJ, Lee KJ, Bang S, et al. Preoperative serum CA 19–9 level as a predictive factor for recurrence after curative resection in biliary tract cancer. Ann Surg Oncol. 2011;18:1651–6.
Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–94.
Soares KC, Kamel I, Cosgrove DP, et al. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3:18–34.
Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691–9.
Sucandy I, Rayman S, Lai EC, et al. Robotic versus laparoscopic left and extended left hepatectomy: an international multicenter study propensity score-matched analysis. Ann Surg Oncol. 2022. https://doi.org/10.1245/s10434-022-12216-6.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81772950 to RYQ, 81773160 to MW, 81702792 to SMX), Hubei Natural Science Foundation (2017CFB467) to MW, Tongji Hospital Clinical Research Flagship Program (2019CR203) to RYQ, and Tongji Hospital Science Fund for Distinguished Young Scholars (2017) to MW, as well as the National Key Research and Development Program of China (2019YFC1315905). The authors are thankful for all the support from grants, and thank all patients and co-investigators at each of the participating centers for collecting and providing the study information. They would also like to thank Editage (www.editage.com) for medical English language editing.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Disclosure
Tingting Qin, Min Wang, Hang Zhang, Jingdong Li, Xiaxing Deng, Yuhua Zhang, Wenxing Zhao, Ying Fan, Dewei Li, Xuemin Chen, Yechen Feng, Siwei Zhu, Zhongqiang Xing, Guangsheng Yu, Jian Xu, Junjie Xie, Changwei Dou, Hongqin Ma, Gangshan Liu, Yue Shao, Weibo Chen, Simiao Xu, Jun Liu, Jianhua Liu, Xinmin Yin, and Renyi Qin declare no conflicts of interest in relation to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Random effects. (a) random effects of centers. (b) Random effects between surgery year. 95% CIs shown
Supplementary Fig. S2
Kaplan–Meier curves for over survival of PHC patients with different Bismuth type. (a) Before propensity score matching; (b) propensity score matching. LS laparoscopic surgery, OP open operation, HR hazard ratio, CI confidence interval
Supplementary Fig. S3
Kaplan–Meier curves for over survival of PHC patients undergoing LS or OP with different Bismuth type. (a) Bismuth I/II type before propensity score matching. (b) Bismuth I/II type after propensity score matching. (c) Bismuth III/IV type before propensity score matching. (d) Bismuth III/IV type after propensity score matching. LS laparoscopic surgery, OP open operation, HR hazard ratio, CI confidence interval
Supplementary Fig. S4
Kaplan–Meier curves for over survival of PHC patients with Bismuth type III-IV patients with R0 resection. (a) Before propensity score matching. (b) propensity score matching. LS laparoscopic surgery, OP open operation, HR hazard ratio, CI confidence interval
Supplementary Table S1
The year-specific survival rate of LS and OP for PHC subgroups in raw data.
Supplementary Table S2
The interoperative and postoperative characteristics according to Bismuth type for PHC patients.
Supplementary Table S3
The interoperative and postoperative characteristics according to surgical method in Bismuth type I/II and Bismuth type III/IV, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, T., Wang, M., Zhang, H. et al. The Long-Term Outcome of Laparoscopic Resection for Perihilar Cholangiocarcinoma Compared with the Open Approach: A Real-World Multicentric Analysis. Ann Surg Oncol 30, 1366–1378 (2023). https://doi.org/10.1245/s10434-022-12647-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12647-1