Abstract
Background
The Multicenter Selective Lymphadenectomy Trial II (MSLT-II) led to a change in the management of tumor-positive sentinel lymph nodes (SLNs) from completion node dissection (CLND) to nodal observation. This study aimed to evaluate prognostic factors for predicting sentinel node basin recurrence (SNBR) using data from MSLT-II trial participants.
Methods
In MSLT-II, 1076 patients were treated with observation. Patients were included in the current study if they had undergone a post-sentinel node basin ultrasound (PSNB-US) within 4 months after surgery. The study excluded patients with positive SLN by reverse transcription-polymerase chain reaction (RT-PCR) or incomplete SLN pathologic data. Primary tumor, patient, PSNB-US, and SLN characteristics were evaluated. Multivariable regression analyses were performed to determine independent prognostic factors associated with SNBR.
Results
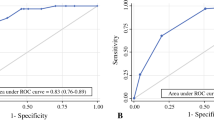
The study enrolled 737 patients: 193 (26.2%) patients with SNBR and 73 (9.9%) patients with first abnormal US. The patients with an abnormal first US were more likely to experience SNBR (23.8 vs. 5.0%). In the multivariable analyses, increased risk of SNBR was associated with male gender (adjusted hazard ratio [aHR], 1.38; 95% confidence interval [CI], 1.00–1.9; p = 0.049), increasing Breslow thickness (aHR, 1.10; 95% CI, 1.01–1.2; p = 0.038), presence of ulceration (aHR, 1.93; 95% CI, 1.42–2.6; p < 0.001), sentinel node tumor burden greater than 1 mm (aHR, 1.91; 95% CI, 1.10–3.3; p = 0.022), lymphovascular invasion (aHR, 1.53; 95% CI, 1.00–2.3; p = 0.048), and presence of abnormal PSNB-US (aHR, 4.29; 95% CI, 3.02–6.1; p < 0.001).
Conclusions
The first postoperative US together with clinical and pathologic factors may play an important role in predicting SNBR.



Similar content being viewed by others
Change history
17 February 2023
A Correction to this paper has been published: https://doi.org/10.1245/s10434-023-13186-z
References
Morton DL, Thompson JF, Cochran AJ, et al. Sentinel node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. https://doi.org/10.1056/NEJMoa060992.
Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel node metastasis in melanoma. N Engl J Med. 2017;376:2211–22. https://doi.org/10.1056/NEJMoa1613210.
Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–67. https://doi.org/10.1016/S1470-2045(16)00141-8.
Broman KK, Richman J, Bhatia S. Evidence and implementation gaps in management of sentinel node–positive melanoma in the United States. Surgery. 2022. https://doi.org/10.1016/j.surg.2021.12.025.
Broman KK, Hughes TM, Bredbeck BC, et al. International center-level variation in utilization of completion lymph node dissection and adjuvant systemic therapy for sentinel lymph node-positive melanoma at major referral centers. Ann Surg. 2022. https://doi.org/10.1097/SLA.0000000000005370.
Bredbeck BC, Mubarak E, Zubieta DG, et al. Management of the positive sentinel lymph node in the post-MSLT-II era. J Surg Oncol. 2020;122:1778–84. https://doi.org/10.1002/jso.26200.
Broman KK, Hughes T, Dossett L, et al. Active surveillance of patients who have sentinel node-positive melanoma: an international, multi-institution evaluation of adoption and early outcomes after the Multicenter Selective Lymphadenectomy Trial II (MSLT-2). Cancer. 2021;127:2251–61. https://doi.org/10.1002/cncr.33483.
Melanoma Cutaneous (version 2.2022). National Comprehensive Cancer Network. Retrieved 17 Dec 2021 at https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf.
Voit C, Mayer T, Kron M, et al. Efficacy of ultrasound B-scan compared with physical examination in follow-up of melanoma patients. Cancer. 2001;91:2409–16. https://doi.org/10.1002/1097-0142(20010615)91:12%3c2409::AID-CNCR1275%3e3.0.CO;2-S.
Blum A, Schlagenhauff B, Stroebel W, Breuninger H, Rassner G, Garbe C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma. Cancer. 2000;88:2534–9. https://doi.org/10.1002/1097-0142(20000601)88:11%3c2534::AID-CNCR15%3e3.0.CO;2-2.
Moehrle M, Blum A, Rassner G, Juenger M. Lymph node metastases of cutaneous melanoma: diagnosis by B-scan and color Doppler sonography. J Am Acad Dermatol. 1999;41:703–9. https://doi.org/10.1016/S0190-9622(99)70004-6.
Hayes AJ, Moskovic E, O’Meara K, et al. Prospective cohort study of ultrasound surveillance of regional lymph nodes in patients with intermediate-risk cutaneous melanoma. BJS Br J Surg. 2019;106:729–34. https://doi.org/10.1002/bjs.11112.
Krüger U, Kretschmer L, Thoms KM, et al. Lymph node ultrasound during melanoma follow-up significantly improves metastasis detection compared with clinical examination alone: a study on 433 patients. Melanoma Res. 2011;21:457–63. https://doi.org/10.1097/CMR.0b013e328348dad3.
Machet L, Nemeth-Normand F, Giraudeau B, et al. Is ultrasound lymph node examination superior to clinical examination in melanoma follow-up? A monocentre cohort study of 373 patients. Br J Dermatol. 2005;152:66–70. https://doi.org/10.1111/j.1365-2133.2004.06262.x.
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801. https://doi.org/10.1056/NEJMoa1802357.
Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–55. https://doi.org/10.1056/NEJMoa1611299.
Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–9. https://doi.org/10.1093/annonc/mdx176.
Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35. https://doi.org/10.1056/NEJMoa1709030.
Broman KK, Bettampadi D, Pérez-Morales J, et al. Surveillance of sentinel node-positive melanoma patients who receive adjuvant therapy without undergoing completion lymph node dissection. Ann Surg Oncol. 2021;28:6978–85. https://doi.org/10.1245/s10434-021-10570-5.
Ho Shon IA, Chung DKV, Saw RPM, Thompson JF. Imaging in cutaneous melanoma. Nucl Med Commun. 2008;29:847–76. https://doi.org/10.1097/MNM.0b013e32830439fb.
Wong SL, Kattan MW, McMasters KM, Coit DG. A nomogram that predicts the presence of sentinel node metastasis in melanoma with better discrimination than the American Joint Committee on Cancer staging system. Ann Surg Oncol. 2005;12:282–8. https://doi.org/10.1245/ASO.2005.05.016.
Lo SN, Ma J, Scolyer RA, et al. Improved risk prediction calculator for sentinel node positivity in patients with melanoma: the Melanoma Institute Australia Nomogram. J Clin Oncol. 2020;38:2719–27. https://doi.org/10.1200/JCO.19.02362.
Acknowledgement
This study was supported by NIH grants CA189163 and CA29605, the Borstein Family Foundation, the Donald L. Morton Research Fund, and the Melamad Family Foundation. The authors would like to acknowledge the following people and institutions on their contributions that lead to the publication of the MSLT-2 trial: John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica (M.B.F., D.S.B.H.), and the Departments of Pathology (A.J.C.), Biomathematics (H.-J.W., D.A.E., R.M.E.), and Medicine (D.A.E.), University of California, Los Angeles — both in California; Melanoma Institute Australia and the University of Sydney, Sydney (J.F.T., O.E.N.), Peter MacCallum Cancer Centre, Melbourne, VIC (M.H.), Princess Alexandra Hospital, Brisbane, QLD (B.M.S.), and Newcastle Melanoma Unit, Waratah, NSW (P.H.) — all in Australia; Huntsman Cancer Institute, Salt Lake City (R.H.A., R.D.N.), and Intermountain Healthcare Cancer Services–Intermountain Medical Center, Murray (T.L.B.) — both in Utah; Istituto Nazionale dei Tumori Napoli, Naples (N.M.), Istituto Europeo di Oncologia, Milan (A.T.), and Istituto Oncologico Veneto–University of Padua, Padua (C.R.R.) — all in Italy; H. Lee Moffitt Cancer Center, Tampa, FL (J.S.Z.); Helsinki University Hospital, Helsinki (T.J.); Dallas Surgical Group, Dallas (P.D.B.); Universitair Medisch Centrum Groningen, Groningen (H.J.H.), and Netherlands Cancer Institute, Amsterdam (M.W.J.M.W.) — both in the Netherlands; Norfolk and Norwich University Hospital, Norwich (M. Moncrieff), and Guy’s and St. Thomas’ NHS Foundation Trust, London (A.M.-R.) — both in the United Kingdom; Swedish Melanoma Study Group–University Hospital Lund, Lund, Sweden (C.I.); University of Michigan, Ann Arbor (M.S.S.); Wake Forest University, Winston-Salem (E.A.L.), and Duke University, Durham (R.S.) — both in North Carolina; Ohio State University, Columbus (D.A.); University of Zurich, Zurich (R.D.), and Centre Hospitalier Universitaire Vaudois, Lausanne (M. Matter) — both in Switzerland; Penn State Hershey Cancer Institute, Hershey (R.I.N.), Thomas Jefferson University (A.C.B.) and Fox Chase Cancer Center (J.M.F.), Philadelphia, and St. Luke’s University Health Network, Bethlehem (D.C.D.) — all in Pennsylvania; Greenville Health System Cancer Center, Greenville, SC (S.D.T.); Sunnybrook Research Institute, Toronto (F.W.), and Tom Baker Cancer Centre, Calgary, AB (G.M.) — both in Canada; University of Washington, Seattle (D.R.B.); Saint Louis University, St. Louis (E.H.); Vanderbilt University (D.B.J., M.C.K.), Nashville, and University of Tennessee, Knoxville (J.M.L.) — both in Tennessee; University Hospital Schleswig–Holstein– Campus Lübeck, Lübeck (P.T.), University Hospital of Würzburg, Würzburg (A.G.), and City Hospital of Nürnberg, Nuremberg (E.S.) — all in Germany; SUNY at Stony Brook Hospital Medical Center, Stony Brook (T.L.H.), Memorial Sloan Kettering Cancer Center, New York (C.E.A.), and Roswell Park Cancer Institute, Buffalo (J.M.K.)— all in New York; Northwestern University Feinberg School of Medicine (J.D.W.) and Rush University Medical Center (S.D.B.), Chicago; University of Wisconsin, Madison (H.B.N.); Tel Aviv Sourasky Medical Center, Tel Aviv, Israel (S.S.); M.D. Anderson Medical Center, Houston (J.E.G.); Johns Hopkins University School of Medicine, Baltimore (L.J.); University of Louisville, Louisville, KY (K.M.M.); Dartmouth–Hitchcock Medical Center, Lebanon, NH (R.J.B.); Hospital Clinic Barcelona, Barcelona (S.V.-S.); and Sentara CarePlex Hospital, Hampton, VA (R.A.H.) [citation #2].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Richard Essner, MD, is on the scientific advisory board and speaker’s bureau for Castle Biosciences and is an advisor for Intra Medical Imaging, Los Angeles, CA, USA. The remaining authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keller, J., Stern, S., Chang, SC. et al. Predicting Regional Lymph Node Recurrence in the Modern Age of Tumor-Positive Sentinel Node Melanoma: The Role of the First Postoperative Ultrasound. Ann Surg Oncol 29, 8469–8477 (2022). https://doi.org/10.1245/s10434-022-12345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12345-y