Abstract
Background
The best management of patients who have unresectable mucinous appendiceal cancer (MAC) with peritoneal spread after a failed attempt at cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is unclear. This study aimed to assess outcomes after systemic chemotherapy (SCT) for patients with unresectable peritoneal metastases from high-grade MAC.
Methods
A single-center retrospective cohort study was conducted using a prospective CRS/HIPEC database. The study included high-grade MAC patients with peritoneal carcinomatosis who were deemed surgical candidates, but had an aborted CRS/HIPEC or only palliative HIPEC due to unresectable disease. Overall survival (OS) was compared.
Results
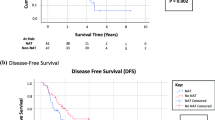
Of 72 identified patients, 20 received SCT and 52 did not (NoCT). The groups were balanced by age (p = 0.299), sex (p = 0.930), histopathologic subtype (p = 0.096), preoperative chemotherapy (p = 0.981), and postoperative major complication rates (p = 0.338). Both groups had extensive disease (median peritoneal cancer index at exploration, 39 vs 39). The median number of cycles was 12 (interquartile range [IQR], 6–15), and the median time between the procedure and SCT was 7 weeks (IQR, 5–10 weeks). The median follow-up period was 65 months. The median OS was significantly higher for the SCT group (26 months; 95 % confidence interval [CI], 10.8–41.5 months) than for the NoCT group (12 months; 95 % CI, 9.6–14.4 months) (p < 0.001), with hazard ratio (HR) of 0.22 (95 % CI, 0.08–0.66; p = 0.007) after adjustment for other factors.
Conclusion
Systemic chemotherapy is associated with improved OS for high-grade MAC patients with unresectable peritoneal metastases who are deemed surgical candidates but underwent an unsuccessful CRS/HIPEC attempt. Further prospective studies with a larger sample are required to identify patient subgroups who benefit the most from SCT.



Similar content being viewed by others
References
Kusamura S, Barretta F, Yonemura Y, et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery. JAMA Surg. 2021;156:e206363.
Guerrero W, Munene G, Dickson PV, et al. Outcome and factors associated with aborted cytoreduction for peritoneal carcinomatosis. J Gastrointest Oncol. 2018;9:664–73.
van Oudheusden TR, Braam HJ, Luyer MD, et al. Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol. 2015;22:1236–42.
Polanco PM, Ding Y, Knox JM, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol. 2015;22:1673–9.
Baron E, Milovanov V, Gushchin V, Sittig M, Neiroda C, Sardi A. Predicting aborted hyperthermic intraperitoneal chemotherapy (AHIPEC) with preoperative tumor and inflammatory markers in potentially resectable appendiceal cancer patients with peritoneal carcinomatosis. Ann Surg Oncol. 2020. https://doi.org/10.1245/s10434-10019-08117-w.
Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer. 2010;116:316–22.
Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2016;122:213–21.
Sugarbaker PH, Bijelic L, Chang D, Yoo D. Neoadjuvant FOLFOX chemotherapy in 34 consecutive patients with mucinous peritoneal carcinomatosis of appendiceal origin. J Surg Oncol. 2010;102:576–81.
Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016;40:14–26.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74.
Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Farquharson AL, Pranesh N, Witham G, et al. A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer. 2008;99:591–6.
Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42.
Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1438–45.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baron, E., Sardi, A., King, M.C. et al. Systemic Chemotherapy for High-Grade Mucinous Appendiceal Cancer with Peritoneal Spread After Unsuccessful CRS/HIPEC. Ann Surg Oncol 29, 6581–6589 (2022). https://doi.org/10.1245/s10434-022-11894-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11894-6