Abstract
Background
Peritoneal metastasis (PM) remains a major obstacle in the treatment of stage IV gastric cancer. This is a dose-escalation study of intraperitoneal (IP) paclitaxel combined with intravenous (IV) fluorouracil, leucovorin, and oxaliplatin (FOLFOX) to determine the recommended phase II dose in gastric cancer patients.
Methods
Patients with gastric adenocarcinoma and PM were enrolled. The recommended phase II dose of IP paclitaxel was determined using the standard “3 + 3” dose escalation with planned doses ranging from 40 to 100 mg/m2. IV FOLFOX was administered on the same day (oxaliplatin 100 mg/m2 (day 1), leucovorin 100 mg/m2 (day 1), fluorouracil 2,400 mg/m2 over 46 hours (day 1)). Both IP and IV regimens were repeated every 2 weeks.
Results
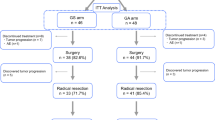
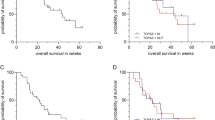
Among the 13 patients, there was no DLT at 40 and 60 mg/m2. Two patients had grade 3 febrile neutropenia at 80 mg/m2, and the recommended phase II dose was 60 mg/m2. Other patients underwent IP paclitaxel and FOLFOX without serious adverse events. Seven patients underwent second-look diagnostic laparoscopy, and the average change in PCI score was −7.0 ± 9.7. Conversion surgery rate was 23.1% (n = 3). The median overall survival was 16.6 months (95% confidence interval, 16.6–N/A), and progression-free survival was 9.6 months (95% confidence interval, 4.7–N/A). All adverse events were tolerable and manageable.
Conclusions
The biweekly regimen of IP paclitaxel and FOLFOX is safe and the recommended dose of IP paclitaxel for a phase II trial is 60 mg/m2.

Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2015;7(32):52307–16. https://doi.org/10.18632/oncotarget.10740.
Thomassen I, Gestel YR, Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–8. https://doi.org/10.1002/ijc.28373.
Shirao K, Boku N, Yamada Y, et al. Randomized phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J Clin Oncol. 2013;43(10):972–80. https://doi.org/10.1093/jjco/hyt114.
Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277–83. https://doi.org/10.1016/s1470-2045(03)01074-x.
Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20(Suppl 1):111–21. https://doi.org/10.1007/s10120-016-0662-9.
Dedrick RL, Myers CE, Bungay PM, DeVita VT. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62(1):1–11.
Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–81. https://doi.org/10.1245/s10434-011-1631-5.
Ukegjini K, Putora PM, Guidi M, et al. Pressurized intraperitoneal aerosol chemotherapy-related clinical trials in the treatment of peritoneal metastases. Oncology. 2021. https://doi.org/10.1159/000516959.
Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC Trial. J Clin Oncol. 2018;36(19):1922–9. https://doi.org/10.1200/jco.2018.77.8613.
Lin R, Chen Y, Zhu J, et al. POF (paclitaxel plus FOLFOX) versus IP PAC (intraperitoneal paclitaxel plus FOLFOX) versus FOLFOX as a first-line treatment in advanced gastric cancer (AGC): update from a multicenter, randomized phase II trial, FNF-004 trial. J Clin Oncol. 2019;37(15_suppl):4035-4035.:https://doi.org/10.1200/jco.2019.37.15_suppl.4035
CTCAE v5. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed August 10, 2021.
Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132–7. https://doi.org/10.1016/j.ejca.2016.03.081.
Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum. 2016;1(2):99–107. https://doi.org/10.1515/pp-2016-0011.
Marchettini P, Stuart AO, Mohamed F, Yoo D, Sugarbaker PH. Docetaxel: pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemoth Pharm. 2002;49(6):499–503. https://doi.org/10.1007/s00280-002-0439-1.
Jacquet P, Sugarbaker PH. Peritoneal carcinomatosis: principles of management. Canc Treat. 1996. https://doi.org/10.1007/978-1-4613-1247-5_4.
van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–40. https://doi.org/10.1056/nejmoa1708618.
Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for Pseudomyxoma peritonei. Ann Surg Oncol. 2007;14(2):484–92. https://doi.org/10.1245/s10434-006-9182-x.
Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–7. https://doi.org/10.1245/s10434-010-1039-7.
Wang Z, Chen JQ, Liu JL, Tian L. Issues on peritoneal metastasis of gastric cancer: an update. World J Surg Oncol. 2019;17(1):215. https://doi.org/10.1186/s12957-019-1761-y.
Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76(5):311–4. https://doi.org/10.1159/000209277.
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. https://doi.org/10.1056/nejmoa052985.
Saito S, Yamaguchi H, Ohzawa H, et al. Intraperitoneal administration of paclitaxel combined with S-1 plus oxaliplatin as induction therapy for patients with advanced gastric cancer with peritoneal metastases. Ann Surg Oncol. 2020. https://doi.org/10.1245/s10434-020-09388-4.
Huang Z, Liu D, Chen X, et al. Deep convolutional neural network based on computed tomography images for the preoperative diagnosis of occult peritoneal metastasis in advanced gastric cancer. Front Oncol. 2020;10:601869. https://doi.org/10.3389/fonc.2020.601869.
Acknowledgments
This study received funding from Boryung Pharmaceutical Co., Ltd, and B. Braun Korea Co., Ltd.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, S.H., Min, SH., Kim, J.W. et al. Safety and Efficacy of Intraperitoneal Paclitaxel Plus Intravenous Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX) for Gastric Cancer with Peritoneal Metastasis. Ann Surg Oncol 29, 5084–5091 (2022). https://doi.org/10.1245/s10434-022-11582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11582-5