Abstract
Background
The development of multimodality treatment, including cytoreductive surgery (CRS) with heated intraperitoneal chemotherapy (HIPEC), has led to promising results in selected patients with peritoneal disease of gastric origin. The aim of this study was to investigate the short- and long-term outcomes of CRS/HIPEC in the treatment of synchronous peritoneal metastasis in gastric cancer.
Methods
The Italian Peritoneal Surface Malignancies Oncoteam—S.I.C.O. retrospective registry included patients with synchronous peritoneal malignancy from gastric cancer submitted to gastrectomy with CRS and HIPEC between 2005 and 2018 from 11 high-volume, specialized centers.
Results
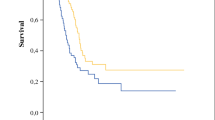
A total of 91 patients with a median age of 58 years (range 26–75) were enrolled. The median overall survival (OS) time for the whole group of patients was 20.2 months (95% confidence interval [CI] 11.8–28.5] and the median recurrence-free survival (RFS) was 7.3 months (95% CI 4–10.6). The completeness of cytoreduction score (CCS) of 0 and Peritoneal Cancer Index (PCI) score of ≤ 6 groups showed a significantly better long-term survival (median OS 40.7 and 44.3 months, respectively) compared with the incomplete resected groups (median OS 10.7 months, p = 0.003) and PCI score of > 6 group (median OS 13.4 months, p = 0.005). A significant difference was observed in the survival rate according to neoadjuvant treatment (untreated patients: 10.7 months, 95% CI 5.1–16.2; treated patients: 35.3 months, 95% CI 2.8–67.8; p = 0.022).
Conclusions
In referral centers, CRS and HIPEC after neoadjuvant treatment significantly improved survival in selected patients. Patients with a PCI score ≤ 6, complete cytoreduction, negative nodal involvements, and negative cytology had encouraging results, showing a clinically meaningful survival.
Similar content being viewed by others
The 5-year survival of gastric cancer (GC) patients with advanced or metastatic disease is dramatically poor, accounting for < 10% of patients.1 The main drawback for curative resection is the peculiar propensity for peritoneal spreading, found in approximately 30% of patients at the time of primary diagnosis.2,3,4 The development of a multimodality treatment strategy, including cytoreductive surgery (CRS) combined with heated intraperitoneal chemotherapy (HIPEC), has recently led to promising results in selected patients with peritoneal disease of gastric origin, thus changing the role of peritoneal disease as a marker for death.5 Since its first description by Sugarbaker et al.6 and Yonemura et al.7 in the 1990s, CRS/HIPEC has progressively shown higher feasibility and efficacy from an oncological point of view. In recent years, similar encouraging results have been obtained by several other research groups,8,9,10 particularly in selected groups of GC patients with oligometastatic peritoneal disease. However, although comprehensive treatment, consisting of CRS combined with HIPEC, seems to be the only strategy to improve the long-term survival of selected GC patients with synchronous PM, there is currently a lack of evidence regarding its clinical value.11,12,13 Recently, several national registries, such as the BIG-RENAPE in France, the Spanish Group of Peritoneal Oncologic Surgery (GECOP), the German HIPEC register, and other national registries, were established to provide, analyze, and share data from multicenter series of patients, allowing an unprecedented exchange of knowledge.13,14,15,16,17,18,19,20
In this study, we describe a nationwide effort undertaken by the Italian Peritoneal Surface Malignancies Oncoteam—S.I.C.O. (Italian Society of Surgical Oncology), with the aim of investigating the short- and long-term outcomes of CRS/HIPEC in the treatment of synchronous PM from GC.
Patients and Methods
Questionnaire and Patient Selection
A questionnaire was created and sent to 11 high-volume and specialized Italian centers involved in CRS and HIPEC, from the Italian Peritoneal Surface Malignancies Oncoteam, in September 2019. The questionnaire included details about patients, perioperative chemotherapeutic regimens, pathologic reports, details of CRS and HIPEC, postoperative complications, and follow-up outcomes. Only patients with pathologically confirmed synchronous PM of GC (according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM classification21) and complete treatment, including gastrectomy with CRS and HIPEC, between 2005 and 2018, were included in the analysis. Exclusion criteria were any distant metastasis (except of the peritoneum) at the time of CRS and HIPEC. All patients provided informed consent for data recording in the registry and were treated according to multidisciplinary recommendations. Due to the retrospective nature of the anonymized data analysis, no Institutional Review Board approval was needed.
Operative Strategy
According to the recommendations of the multidisciplinary team, patients underwent similar management with CRS and HIPEC in combination with neoadjuvant chemotherapy (NACT) whenever possible. After completion of NACT, tumor response was assessed using the new Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.1.22 In all centers, CRS and HIPEC were performed by a multidisciplinary team (surgeons, anesthesiologists, and operating room staff) specialized in peritoneal surgery and in the management of intraoperative chemotherapy. The extent of PM was assessed using the Peritoneal Cancer Index (PCI)23 immediately before CRS procedures, along with lavage peritoneal sampling; however, lavage cytology was not routinely performed in all patients prior to NACT and prior to CRS. Definitive CRS was performed in accordance with the techniques described by Sugarbaker 24, aiming at achieving complete cytoreduction. To reach this goal, patients underwent total or subtotal gastrectomy with D2 lymphadenectomy25 to remove the primary tumor, followed by peritonectomy procedures and visceral resections on demand in order to remove all visible peritoneal implants. After cytoreduction, and before HIPEC, all restorative anastomoses were completed and surgical radicality was determined according to the completeness of cytoreduction score (CCS).26 CCS-0 indicates no visible residual tumor and CCS-1 indicates residual tumor nodules ≤ 2.5 mm, while CCS-2 and CCS-3 indicate residual tumor nodules between 2.5 mm and 2.5 cm, and > 2.5 cm, respectively.27 HIPEC was performed using different protocols, depending on the center’s preferences, that differ in exposure technique, time, drugs, temperature. Postoperative mortality was defined as death within 90 days of surgery, while postoperative morbidity was recorded and scored according to the Clavien–Dindo classification system.28 Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE V4.0). After hospital discharge and complete recovery from surgery, patients received systemic chemotherapy possibly combined with biologic therapy according to their general status and the center’s protocols.
Follow-Up
Patients were regularly followed-up after surgery, either in the surgical or oncological department, with blood tests (including tumor markers) and computed tomography every 3 months for the first 2 years, every 6 months from years 3–5, and yearly thereafter, or on demand, at any time, according to clinical status.
Study Endpoints and Definition
The primary endpoints of this study were overall survival (OS), calculated from the date of CRS and HIPEC to the date of death by any cause or last contact, and recurrence-free survival (RFS), calculated from the day of CRS and HIPEC until the date of locoregional or distant recurrence. The secondary endpoints were analysis of morbimortality and prognostic factors for survival.
Statistical Analysis
Descriptive statistics were reported as median (minimum and maximum values) or frequency (percentage). The estimated median follow-up time was obtained according to the reverse Kaplan–Meier method.29 OS and RFS analyses were performed using the Kaplan–Meier estimation method and compared using the log-rank test. All variables, which showed a p-value below 0.05 in univariate analysis, were included in the Cox regression model. Cox proportional hazards regression was used to calculate the hazard ratios (HRs) and 95% confidence intervals for the risk factors. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS version 26.0 software package for Mac (IBM Corporation, Armonk, NY, USA).
Results
Patient and Treatment Characteristics
Of the 212 patients in the national multicentric database, 121 patients were excluded since, among them, 64 (30.2%) were submitted to prophylactic HIPEC, 31 patients (14.6%) were treated for metachronous peritoneal disease and 26 patients (12.3%) were submitted to laparoscopic HIPEC with palliative intent. A complete dataset of 91 patients fulfilling the selection criteria was available for this study. At preoperative work-up, a median PCI of 7 (range 2–34) was found. No data regarding the number of patients submitted to staging laparoscopy were available. In total, 60 (65.9%) patients received a median of 6 (1–14) cycles of preoperative chemotherapy using epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) in the majority of patients. During NACT, 8 patients (8.8%) had grade 3 neutropenia and 12 (10.9%) patients had grade 3 anemia. These 20 patients received growth factor and only 5 patients needed NACT dose reductions. After NACT, restaging according to the RECIST criteria showed that 2 patients (3.3%) had complete response, 30 cases (50%) had partial response, and 28 cases (46.7%) presented with stable disease. Table 1 summarizes the clinicopathologic characteristics of selected patients. Peritoneal cytology results were positive for metastasis in 33 patients (36.3%), negative in 51 patients (56%), and non-diagnostic in 7 patients (7.7%). Interestingly, among 60 patients treated with NACT, only 3 patients (5%) experienced free peritoneal cancer (PC) cells.
Cytoreductive Surgery
At surgery, the median PCI was 6 (range 1–39). Forty-nine patients (53.8%) showed a median PCI of ≤ 6 and 42 patients (46.2%) showed a median PCI of > 7. The incidence and type of resection of additional organs to reach a CCS-0 status, as well as other operative details, are listed in Table 2.
Heated Intraperitoneal Chemotherapy
The majority of centers performed a closed HIPEC technique (75 patients, 82.4%). The median temperature was 42 °C (range 40–42) and the mean duration of intra-abdominal chemotherapy was 60 min (range 30–90). Cisplatin, mitomycin C, and oxaliplatin were mostly used as HIPEC drugs. Two drugs (cisplatin and mitomycin C) were simultaneously administered in 36 patients (39.6%). Single-drug HIPEC with mitomycin C was administered in 11/55 patients (20%), cisplatin was administered in 20/55 patients (36.4%), and oxaliplatin was administered in 24/55 patients (43.6%). The median dose of cisplatin, mitomycin C, and oxaliplatin was 141 mg/m2 (range 77–350), 24.5 mg/m2 (range 10–54), and 360 mg/m2 (range 360–540), respectively.
Postoperative Outcomes
After surgery, all patients were transferred to the intensive care unit for recovery until all vital signs were stabilized. HIPEC induced toxicity in 8 patients (8.8%): grade 1–2 acute kidney injury in two patients, grade 3 thrombopenia in two patients, and grade 3 leukopenia in four patients, promptly reversed by medical treatment. Clavien–Dindo classification grade 3b or higher occurred in 27 patients (29.7%), including six deaths (6.6% 90-day mortality); two of these patients developed acute myocardial infarction, one patient had a massive hemoperitoneum requiring an unsuccessful surgical intervention, and, in the remaining three cases, the cause of death was respiratory failure secondary to acute respiratory distress syndrome, septic shock in the context of anastomotic complication, and acute liver failure, respectively. After surgery, 71 patients (78%) completed systemic chemotherapy, while the remaining patients were not able to start the treatment due to postoperative complications that delayed discharge and recovery. Table 3 summarizes the main postoperative outcomes in the overall patients.
Survival Analysis
The median follow-up was 47 months and no patients were lost to follow-up. The median OS time for the entire group of patients was 20.2 months (95% CI 11.8–28.5), with 1-, 3-, and 5-year OS rates of 62%, 44%, and 20.4%, respectively (Fig. 1a). The median RFS was 7.3 months (95% CI 4–10.6), with 1- and 3-year RFS rates of 14.3% and 4.8%, respectively (Fig. 1b). We ran survival analysis, grouping the PCI into two categories: PCI ≤ 6 (group 1, n = 49), and PCI from 7 to 39 (group 2, n = 42). In group 1, the median OS was 44.3 months (95% CI 16.4–72.1) and in group 2, the OS was 13.4 months (95% CI 6.2–20.5), with 5-year OS of 33.2% and 5.5%, respectively (p = 0.005) (Fig. 2a). The CCS-0 group showed better long-term survival compared with the incomplete resected group, reaching a high level of significance (p < 0.003). The median survival (months) was 40.7 (95% CI 11.7–69.7) for the CCS-0 group and 10.7 (95% CI 4.4–17) for the CCS > 0 group (Fig. 2b). The 5-year OS rate was higher for the CCS-0 group than the CCS > 0 group (25.9% vs. 5.9%). A significant difference was also observed in the survival rate according to NACT (untreated patients: 10.7 months, 95% CI 5.1–16.2; treated patients: 35.3 months, 95% CI 2.8–67.8; p = 0.022) (Fig. 2c). Finally, the median OS for patients with positive peritoneal cytology was worse than that of patients without free peritoneal metastatic cells (10.3 months [95% CI 3.9–17.6] vs. 44.3 months [95% CI 14.7–73.9]; p = 0.023) (Fig. 2d). We also investigated the differences in OS between patients according to other parameters (Table 4). The increase in mortality risk was nearly twofold in patients with PCI > 6 and CCS > 0 compared with those with PCI ≤ 6 and CCS-0. After adjusting for NACT, patients with PCI > 6 had a significantly increased risk of mortality compared with those with PCI ≤ 6. On the other hand, patients with CCS > 0 still had an increased risk but this was not statistically significant (Table 5). Further adjustments for peritoneal cytology and nodal status were made but had little effect on the HR.
Discussion
The Italian Peritoneal Surface Malignancies Oncoteam network shows encouraging results, since, among a total of 91 enrolled patients, median OS was 20.2 months, with a 5-year OS rate of 20.4%. Several retrospective studies have identified predictive factors for patient selection regarding patients treated with CRS and HIPEC in the curative treatment of synchronous PM from GC.10,11,18,30 Nevertheless, the limited number of patients per study, as well as the consistent rate of incomplete or noncurative surgeries due to high peritoneal tumor burden, could affect the results.3,7,11,20,31,32,33,34,35,36
Peritoneal Cancer Index
It is well-established that the lower the quantitative expression of tumor burden, expressed as PCI, the greater the chance to obtain complete CRS.37 However, if on the one hand the role of PCI as a prognostic factor is clear, on the other hand the cut-off for favorable prognosis is still debated.3,11,20,30,38 French and Japanese groups support a cut-off level of ≤ 6 or 7 according to their retrospective series,11,39 even if a cut-off of 12 does not seem unreasonable.10,11,40 In 81 patients with GC and PC from five French institutions treated with complete CRS and HIPEC, Chia et al.37 reported a median OS for patients with PCI < 7 of 26.4 months versus 10.9 months for patients with PCI ≥ 7. This same PCI cut-off also proved significantly prognostic for survival in the study by Yonemura41 (median OS of 33 months for PCI < 7 vs. 13 months for higher PCI scores), and, more recently, in a multicenter study of Spanish Group of Peritoneal Oncologic Surgery (patients with PCI ≤ 6 had a median OS of 26.1 months vs. 18.1 months for patients with PCI > 6).8 On the other hand, Coccolini et al.40 proposed a PCI score of 12 as the cut-off point for selecting patients with GC and PC for CRS and HIPEC. Survival was significantly better for PCI < 12, with a 3-year OS rate of 33% for PCI ≤ 6, 18% for PCI from 7 to 12, and 0% for PCI ≥ 13. Taking this evidence into consideration, in our cohort, patients with PCI ≤ 6 (53.8%) had a median OS of 44.3 months and a 5-year OS of 33.2%. Additionally, the mortality risk was doubled in patients with PCI > 6 compared with patients with PCI ≤ 6. Based on these results, we propose a PCI score of 6 as the cut-off point for selecting patients with PM from GC for CRS and HIPEC, excluding such treatment in patients with a higher PCI.
Completeness of Cytoreduction Score
In their systematic review of 17 studies on the treatment outcomes of CRS and HIPEC for PM from GC, Chia et al.32 reported a median OS ranging from 6.6 to 15.8 months, with a 5-year survival rate of between 6% and 31%. Our favorable OS can be partly explained by the high quality of surgery since we obtained complete cytoreduction (CCS-0) in 80.2% of enrolled patients. Furthermore, all patients were treated at Italian Peritoneal Surface Malignancies Oncoteam centers, which include only recognized high-volume Italian centers with specific competence in peritoneal and gastric malignancies. Interestingly, our findings also confirm the crucial role of complete cytoreduction on survival outcomes. The CCS-0 group showed a significantly better long-term survival (median OS 40.7 months) compared with the incomplete resected groups (median OS 10.7 months). Similarly, in a series of 159 patients from 15 institutions that included patients with gastric PC treated with CRS and HIPEC, Glehen et al.11 reported a median OS of 15 months for CCS-0 and extremely low benefit for CCS ≥ 1 (median OS 6–8 months). In a recent large propensity score analysis of 277 patients by Glehen et al.,13 minimal residual disease (CCS-1) was associated with worse prognosis, resulting in a 5-year OS of 24.8% in CCS-0, compared with only 6.2% in the CCS-1 group. Accordingly, our survival analysis is in line with these findings. However, completeness of cytoreduction was found to be a prognostic factor in several other studies,3,8,20,37 suggesting CCS-0 as mandatory before a HIPEC procedure.
Neoadjuvant Chemotherapy
The poor survival rate of patients who experienced incomplete or noncurative surgery due to a high PCI may be improved by perioperative chemotherapies. Accordingly, Valle et al.42 reported that CCS-0 can be realized in <30% of patients managed with upfront surgery, suggesting that patients with a PCI higher than the cut-off level at preoperative laparoscopy should be treated by NACT in order to reduce the tumor burden and accrue good prognosis after CRS and HIPEC. Currently, the Italian Research Group for Gastric Cancer (GIRCG) guidelines for the diagnosis and treatment of GC recommend neoadjuvant treatment for GC T ≥ 3 and/or with metastatic nodes on preoperative work-up.43 Nevertheless, there are still limited data about the effect of preoperative chemotherapy in patients with peritoneal metastases (PM) of GC. Our study was able to demonstrate a potentially beneficial effect of NACT on OS in patients eligible for CRS and HIPEC. Interestingly, among 60 patients treated with NACT, 51 (85%) presented with negative cytology and only 3 (5%) presented with free PC cells. However, 34.1% of patients were not treated with NACT and the reasons why were not stated in our registry, therefore remaining unclear. Potential reasons for this issue could be patients being operated before the introduction of NACT in the Italian guidelines. A significant difference was observed in the median OS according to NACT between both groups (untreated patients: 10.7 months; treated patients: 35.3 months). We also confirmed NACT favorable prognostic effect on OS in a Cox regression hazard model (HR 1.97, p < 0.049). Interestingly, the median OS in patients receiving four or fewer cycles of NACT was lower when compared with patients receiving more than five cycles (35.3 and 50.5 months, respectively), with no statistical significance. The number of NACT cycles actually represents an unresolved issue, since conflicting results have been published in the literature. In a randomized phase III study, Yang et al.12 reported improved OS for a cohort with more than six cycles, while Rau et al.10 demonstrated a potential negative effect of prolonged preoperative intravenous chemotherapy on OS. The differences could be explained on the basis of the heterogeneity of NACT drug protocols. We administered the protocol consisting of epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) in the majority of patients. Preoperative treatment in the German group, instead, included a triple combination of oxaliplatin, leucovorin, and docetaxel, with taxane (FLOT).44 Obviously, there could be other occult factors influencing long-term survival in these patients, such as the differences from a molecular as well as biological point of view.
Lymph Node Metastasis and Positive Cytology
Patients at risk of developing PM after curative resection for GC include those with nodal invasion and those with positive cytology.45,46,47 It is well-established that the impact of lymph node metastasis on OS, as well as tumor recurrence for GC patients, is extremely negative.48,49 Nevertheless, there is still limited evidence about the role of nodal involvement in patients with PM in GC treated with CRS and HIPEC. On this topic, Rau et al.10 reported a significantly improved median OS for N0 (26.7 months) rather than N+ (9.3 months) in a cohort of 58 patients treated with CRS and HIPEC. Accordingly, our data were able to demonstrate a negative effect of nodal involvement on survival outcomes. The median OS for patients with positive nodes was worse than that of patients without metastatic lymph nodes (13.7 months vs. 60 months; p = 0.016). It is likely the minor tumor burden of nodal-negative patients, from a systemic point of view, could lead to better response after CRS and HIPEC; however, further research into the genetic, molecular, and biological aspects of PM from GC may help identify tumor-specific markers associated with prognosis.
An important finding of our analysis is the significant difference between patients with positive peritoneal cytology at CRS (10.3 months) and patients without free peritoneal metastatic cells (44.3 months), resulting in positive cytology being a negative prognostic factor in Cox regression hazard models. However, in spite of this interesting evidence, the published data are scarce and it appears challenging to provide a robust explanation. It is well-recognized that patients with positive cytology have an 81% risk of peritoneal disease after curative surgery, as opposed to 45% for those with negative cytology.50 Nonetheless, to the best of our knowledge, no studies have been conducted investigating the role of circulating metastatic cells in patients with PM from GC. We highlight the favorable impact of negative cytology on peritoneal metastatic patients as a result of GC, postulating that these patients might have less malignant tumor biological characteristics and therefore benefit from CRS and HIPEC. Additionally, further studies exploring the application of new therapeutic tools, such as extensive intraoperative peritoneal lavage (EIPL),51 as well as bidirectional chemotherapy,52 are needed to help us better select which patients may be suitable for CRS and HIPEC. Interestingly, in the last century, attention has been focused on repeated intraperitoneal chemotherapy (RIPEC) using taxanes at normothermic conditions, mainly in the Eastern Countries.53 Even if the mechanism for shrinkage of peritoneal tumors has not been fully understood, repeated doses of intraperitoneal taxanes combined with systemic chemotherapy as neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) resulted in an impressive improvement of survival outcomes (15.1–30.5 months) in connection with manageable toxicities.54,55,56 This tool may be a promising strategy for the management of peritoneal disease for GC and this contextual insight could be useful for developing hypotheses for further study.
Limitations
One major limitation of our multicentric collaborative study is the heterogeneity of the selection criteria and management between institutions performing CRS and HIPEC. Additionally, the multicentric and retrospective nature of the data may affect some subjective results, such as intraoperative PCI and CCS. Nonetheless, this is one of the most important national registries, including only data from recognized high-volume Italian centers with specific competence in peritoneal and gastric malignancies, published to date.
Conclusions
Although GC with PC still has poor prognosis, CRS and HIPEC after NACT, in referral centers, significantly improved survival in selected patients. Patients with a PCI score ≤ 6, complete cytoreduction, negative nodal involvements, and negative cytology experienced encouraging results, showing a clinically meaningful survival.
References
Marano L, Chiari R, Fabozzi A, et al. c-Met targeting in advanced gastric cancer: an open challenge. Cancer Lett. 2015;365(1):30–6. https://doi.org/10.1016/j.canlet.2015.05.028.
Gretschel S, Siegel R, Estévez-Schwarz L, Hünerbein M, Schneider U, Schlag PM. Surgical strategies for gastric cancer with synchronous peritoneal carcinomatosis. Br J Surg. 2006;93(12):1530–5. https://doi.org/10.1002/bjs.5513.
Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92(3):370–5. https://doi.org/10.1002/bjs.4695.
Ikoma N, Blum M, Chiang YJ, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23(13):4332–7. https://doi.org/10.1245/s10434-016-5409-7.
Gamboa AC, Winer JH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer. Cancers (Basel). 2019;11(11):1662. https://doi.org/10.3390/cancers11111662.
Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. In: Banzet P, Holland JF, Khayat D, Weil M (eds). Proceedings on the 3rd international congress on neo-adjuvant chemotherapy. Paris: Springer; 1989. pp. 272-75. https://doi.org/10.1007/978-2-8178-0782-9_67
Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119(4):437–44. https://doi.org/10.1016/S0039-6060(96)80145-0.
Rihuete Caro C, Manzanedo I, Pereira F, Carrion-Alvarez L, Serrano Á, Pérez-Viejo E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44(11):1805–10. https://doi.org/10.1016/j.ejso.2018.06.036.
Yarema R, Mielko J, Fetsych T, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: a retrospective cooperative Central-Eastern European study. Cancer Med. 2019;8(6):2877–85. https://doi.org/10.1002/cam4.2204.
Rau B, Brandl A, Thuss-Patience P, et al. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer. 2019;22(6):1226–37. https://doi.org/10.1007/s10120-019-00969-1.
Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–7. https://doi.org/10.1245/s10434-010-1039-7.
Yang X-J, Huang C-Q, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–81. https://doi.org/10.1245/s10434-011-1631-5.
Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol. 2019;37(23):2028–40. https://doi.org/10.1200/JCO.18.01688.
Rovers KP, Simkens GA, Vissers PA, et al. Survival of patients with colorectal peritoneal metastases is affected by treatment disparities among hospitals of diagnosis: a nationwide population-based study. Eur J Cancer. 2017;75:132–40. https://doi.org/10.1016/j.ejca.2016.12.034.
Verwaal VJ, Rau B, Jamali F, et al. Registries on peritoneal surface malignancies throughout the world, their use and their options. Int J Hyperth. 2017;33(5):528–33. https://doi.org/10.1080/02656736.2017.1315178.
Sugarbaker PH. The Seven Best from PSOGI 2016. Ann Surg Oncol. 2017;24(4):870–4. https://doi.org/10.1245/s10434-017-5793-7.
Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27(36):6237–42. https://doi.org/10.1200/JCO.2009.23.9640.
Rau B, Brandl A, Piso P, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23(1):11–22. https://doi.org/10.1007/s10120-019-00978-0.
Manzanedo I, Pereira F, Rihuete Caro C, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: multicenter study of spanish group of peritoneal oncologic surgery (GECOP). Ann Surg Oncol. 2019;26(8):2615–21. https://doi.org/10.1245/s10434-019-07450-4.
Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139(1):20–6. https://doi.org/10.1001/archsurg.139.1.20.
Brierley J, Gospodarowicz MK, Wittekind C (eds). TNM classification of malignant tumours. https://www.wiley.com/en-it/TNM+Classification+of+Malignant+Tumours,+8th+Edition-p-9781119263579. Accessed 4 Jul 2020.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. https://doi.org/10.1016/j.ejca.2008.10.026
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74. https://doi.org/10.1007/978-1-4613-1247-5_23.
Deraco M, Glehen O, Helm CW, Morris DL, Speeten K. Cytoreductive surgery & perioperative intraperitoneal chemotherapy for peritoneal surface malignancy: textbook and video atlas. Woodbury, CT: Cine-Med; 2012.
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1-19. doi:https://doi.org/10.1007/s10120-016-0622-4
González-Moreno S, Kusamura S, Baratti D, Deraco M. Postoperative residual disease evaluation in the locoregional treatment of peritoneal surface malignancy. J Surg Oncol. 2008;98(4):237–41. https://doi.org/10.1002/jso.21072.
Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10(8):863–9. https://doi.org/10.1245/aso.2003.01.018.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Sathish N, Wu CA. Let’s Flip: An Approach to Understand Median Follow-up by the Reverse Kaplan-Meier Estimator from a Statistical Programmer’s Perspective. 2019. Available at: https://www.pharmasug.org/proceedings/2019/ST/PharmaSUG-2019-ST-081.pdf.
Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol. 2016;42(8):1123–31. https://doi.org/10.1016/j.ejso.2016.03.016.
Fujimoto S, Shrestha RD, Kokubun M, et al. Positive results of combined therapy of surgery and intraperitoneal hyperthermic perfusion for far-advanced gastric cancer. Ann Surg. 1990;212(5):592–6. https://doi.org/10.1097/00000658-199011000-00005.
Chia CS, You B, Decullier E, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 2016;23(6):1971–9. https://doi.org/10.1245/s10434-015-5081-3.
Yonemura Y, Fujimura T, Fushida S, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg. 1991;15(4):530–5. https://doi.org/10.1007/BF01675656.
Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8(4):454–63. https://doi.org/10.1016/j.gassur.2003.12.014.
Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008;34(11):1246–52. https://doi.org/10.1016/j.ejso.2007.12.003.
Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol. 2014;21(5):1448–55. https://doi.org/10.1245/s10434-013-3327-5.
Chia CS, Seshadri RA, Kepenekian V, Vaudoyer D, Passot G, Glehen O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: a systematic review. Pleura Peritoneum. 2016;1(2):67–77. https://doi.org/10.1515/pp-2016-0010.
Yonemura Y, Endou Y, Shinbo M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: selection for cytoreductive surgery. J Surg Oncol. 2009;100(4):311–6. https://doi.org/10.1002/jso.21324.
Yonemura Y, Elnemr A, Endou Y, et al. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2(2):85–97. https://doi.org/10.4251/wjgo.v2.i2.85.
Coccolini F, Catena F, Glehen O, et al. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur J Surg Oncol. 2015;41(7):911-19. https://doi.org/10.1016/j.ejso.2015.03.231
Yonemura Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2(2):85. https://doi.org/10.4251/wjgo.v2.i2.85.
Valle M, Federici O, Garofalo A. Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy, and Role of Laparoscopy in Diagnosis, Staging, and Treatment. Surg Oncol Clin N Am. 2012;21(4):515–31. https://doi.org/10.1016/J.SOC.2012.07.005.
De Manzoni G, Marrelli D, Luca Baiocchi G, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2017;20(1):20–30. https://doi.org/10.1007/s10120-016-0615-3.
Al-Batran S-E, Homann N, Pauligk C, et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3(9):1237. https://doi.org/10.1001/JAMAONCOL.2017.0515.
Wu CW, Lo SS, Shen KH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 2003;27(2):153–8. https://doi.org/10.1007/s00268-002-6279-7.
Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K. Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol. 2010;17(2):455–60. https://doi.org/10.1245/s10434-009-0764-2.
Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: An ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9(2):678–85.
Seyfried F, von Rahden BH, Miras AD, et al. Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin - a longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer. 2015;15(1):1–10. https://doi.org/10.1186/s12885-015-1081-8.
Smyth EC, Fassan M, Cunningham D, et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34(23):2721–7. https://doi.org/10.1200/JCO.2015.65.7692.
Yonemura Y, Endou Y, Sasaki T, et al. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol. 2010;36(12):1131–8. https://doi.org/10.1016/j.ejso.2010.09.006.
Kuramoto M, Shimada S, Ikeshima S, et al. Extensive Intraoperative Peritoneal Lavage as a Standard Prophylactic Strategy for Peritoneal Recurrence in Patients with Gastric Carcinoma. Ann Surg. 2009;250(2):242–6. https://doi.org/10.1097/SLA.0b013e3181b0c80e.
Yonemura Y. A new bidirectional intraperitoneal and systemic induction chemotherapy (BISIC) for the peritoneal metastasis from gastric cancer in neoadjuvant setting. Integr Cancer Sci Ther. 2014;1(2):26–9. https://doi.org/10.15761/ICST.1000106.
Kitayama J, Ishigami H, Yamaguchi H, et al. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2018;2(2):116–23. https://doi.org/10.1002/ags3.12060.
Cho H, Ryu M-H, Kim K-P, et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer. 2017;20(6):970–7. https://doi.org/10.1007/s10120-017-0710-0.
Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21(2):539–46. https://doi.org/10.1245/s10434-013-3208-y.
Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20(Suppl 1):128–34. https://doi.org/10.1007/s10120-016-0684-3.
Funding
Open access funding provided by Università degli Studi di Siena within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosures
Luigi Marano, Daniele Marrelli, Paolo Sammartino, Daniele Biacchi, Luigina Graziosi, Elisabetta Marino, Federico Coccolini, Paola Fugazzola, Mario Valle, Orietta Federici, Dario Baratti, Marcello Deraco, Andrea Di Giorgio, Antonio Macrì, Enrico Maria Pasqual, Massimo Framarini, Marco Vaira, and Franco Roviello have no competing interests or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marano, L., Marrelli, D., Sammartino, P. et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of ‘Italian Peritoneal Surface Malignancies Oncoteam—S.I.C.O.’. Ann Surg Oncol 28, 9060–9070 (2021). https://doi.org/10.1245/s10434-021-10157-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10157-0