Abstract
Background
The effects of video-assisted thoracoscopic surgery (VATS) and traditional thoracotomy with respect to patient-reported outcomes (PROs) have only been assessed for early-stage lung cancer. This study was a longitudinal PRO assessment to compare the effects of these surgeries for locally advanced (stage II and III) lung cancer from the patients’ perspective.
Methods
We investigated lung cancer patients from a previous prospective, multicentre study. Longitudinal data of clinical characteristics and PROs were collected. PROs were obtained preoperatively, daily in the hospital postoperatively, and weekly up to 4 weeks after discharge or the beginning of postoperative adjuvant therapy. Symptoms and impact on daily functioning and quality of life (QOL) were assessed by using the MD Anderson Symptom Inventory for lung cancer and a single-item QOL scale. Trajectories of PROs over the investigation period were compared.
Results
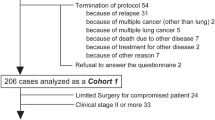
Overall, 117 primary lung cancer patients (stage II or III), including 63 and 54 patients who underwent VATS and traditional thoracotomy, respectively, were included. During postoperative hospitalization, VATS patients reported milder disturbed sleep (p = 0.048), drowsiness (p = 0.008), and interference with activity (p = 0.001), as well as better work ability (p < 0.0001), walking ability (p < 0.0001), and life enjoyment (p = 0.004). Post-discharge, VATS patients had less distress (p = 0.039), milder pain (p = 0.006), better work ability (p = 0.001), and better QOL (p = 0.047).
Conclusions
Locally advanced lung cancer patients who underwent VATS had lower postoperative symptom burden, less daily function interference, and better QOL than those who underwent thoracotomy.



Similar content being viewed by others
Change history
24 September 2021
A Correction to this paper has been published: https://doi.org/10.1245/s10434-021-10798-1
References
Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg. 2013;145:514–20.
Cioffi U, De Simone M, Baisi A. Is video-assisted thoracic lobectomy safe and successful for locally advanced non-small cell lung cancer? J Thorac Cardiovasc Surg. 2013;146:1302–3.
NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. [Internet]. Version 6. National Comprehensive Cancer Network; 2020. Accessed 30 June 2020. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Wang Z, Pang L, Tang J, et al. Video-assisted thoracoscopic surgery versus muscle-sparing thoracotomy for non-small cell lung cancer: a systematic review and meta-analysis. BMC Surg. 2019;19:144.
Long H, Tan Q, Luo Q, et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg. 2018;105:386–92.
Cao C, Manganas C, Ang SC, Peeceeyen S, Yan TD. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg. 2013;16:244–9.
Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–44.
Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–9.
Mehran R, Baber U, Dangas G. Guidelines for patient-reported outcomes in clinical trial protocols. JAMA. 2018;319:450–1.
Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs. clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–32.
Molassiotis A, Uyterlinnde W, Hollen PJ, Sarna L, Palmer P, Krishnasamy M. Supportive care in lung cancer: milestones over the past 40 years. J Thorac Oncol. 2015;10:10–8.
Glied SA, Frank RG. Care for the vulnerable vs. cash for the powerful—Trump's pick for HHS. N Engl J Med. 2017;376:103–5.
Rotenstein LS, Huckman RS, Wagle NW. Making patients and doctors happier—the potential of patient-reported outcomes. N Engl J Med. 2017;377:1309–12.
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
Ma SJ, Oladeru OT, Miccio JA, Iovoli AJ, Hermann GM, Singh AK. Association of timing of adjuvant therapy with survival in patients with resected stage I to II pancreatic cancer. JAMA Netw Open. 2019;2:e199126.
Licht PB, Schytte T, Jakobsen E. Adjuvant chemotherapy compliance is not superior after thoracoscopic lobectomy. Ann Thorac Surg. 2014;98:411–5; discussion 415–6.
Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–65.
Salazar MC, Rosen JE, Wang Z, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–9.
Dai W, Xie S, Zhang R, et al. Developing and validating utility parameters to establish patient-reported outcome-based perioperative symptom management in patients with lung cancer: a multicentre, prospective, observational cohort study protocol. BMJ Open. 2019;9:e030726.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46.
Wang XS, Wang Y, Guo H, et al. Chinese version of the M. D. Anderson Symptom Inventory: validation and application of symptom measurement in cancer patients. Cancer. 2004;101:1890–901.
Sloan JA, Loprinzi CL, Kuross SA, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol. 1998;16:3662–73.
Kirkova J, Davis MP, Walsh D, et al. Cancer symptom assessment instruments: a systematic review. J Clin Oncol. 2006;24:1459–73.
Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M.D. Anderson Symptom Inventory. Oncologist. 2011;16:217–27.
Shi Q, Trask PC, Wang XS, et al. Does recall period have an effect on cancer patients’ ratings of the severity of multiple symptoms? J Pain Symptom Manag. 2010;40:191–9.
Bhagat R, Bronsert MR, Henderson WG, et al. Analysis of discharge destination after open versus minimally invasive surgery for lung cancer. Ann Thorac Surg. 2020;109:375–82.
Chai T, Lin Y, Kang M, Lin J. Thoracotomy versus video-assisted thoracoscopic resection of lung cancer: a protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14646.
Wolf A, Liu B, Leoncini E, et al. Outcomes for thoracoscopy versus thoracotomy not just technique dependent: a study of 9,787 patients. Ann Thorac Surg. 2018;105:886–91.
Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: an alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg. 2015;20:813–9.
Shi Q, Wang XS, Vaporciyan AA, Rice DC, Popat KU, Cleeland CS. Patient-reported symptom interference as a measure of postsurgery functional recovery in lung cancer. J Pain Symptom Manage. 2016;52:822–31.
Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg. 2015;150(613–9):e2.
Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35:20–30.
Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;16–21.
Abrams BA, Murray KA, Mahoney K, et al. Post-discharge pain management after thoracic surgery—a patient-centered approach. Ann Thorac Surg. 2020;110:1714–21.
Fang L, Wang L, Wang Y, Lv W, Hu J. Video assisted thoracic surgery vs. thoracotomy for locally advanced lung squamous cell carcinoma after neoadjuvant chemotherapy. J Cardiothorac Surg. 2018;13:128.
Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–78.
Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–62.
Mendoza TR, Kehl KL, Bamidele O, et al. Assessment of baseline symptom burden in treatment-naive patients with lung cancer: an observational study. Support Care Cancer. 2019;27:3439–47.
Acknowledgement
The authors thank Wenhong Feng from the Department of Thoracic and Cardiovascular Surgery, Jiangyou People’s Hospital; Yuanqiang Zhang from the Department of Cardiothoracic Surgery, Zigong First People's Hospital; Rui Zhang from the Department of Thoracic Surgery, Chengdu Seventh People's Hospital; and Xiaoqing Liao from the Department of Cardiothoracic Surgical Oncology, Dazhu County People's Hospital, Dazhu County, for their contributions to the data collection of this study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81872506).
Author information
Authors and Affiliations
Contributions
Study concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors Drafting of the article: XW. Revising the article critically for important intellectual content: All authors. Final approval of the version to be published: All authors. Statistical analysis: HY Obtained funding: QS. Administrative, technical, or material support: QL, QS. Study supervision: QL, QS. XW and HY had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Disclosure
The authors have no conflict of interest to declare.
Previous Communication
Partial preliminary results of this article were orally presented by the first author at the 23rd Annual Meeting of the Chinese Society of Clinical Oncology (CSCO), which was held virtually from 19 to 26 September 2020. Partial preliminary results of further refinement and revision of this article were briefly presented by the first author at the 27th Annual Conference of the International Society for Quality of Life Research (ISOQOL), which was held virtually from 19 to 23 October 2020 (Abstract #: B201.10).
Research Transparency
The authors are willing to share data, analytic methods, and study materials related to this article with other researchers provided that all of the above will not be used for commercial or profit purposes. Other researchers can contact the corresponding author of this article by email and indicate the required research materials and purpose. We will be glad to provide relevant materials for this study after approval and discussion. The data of this research was from a longitudinal PRO cohort study which was preregistered in an independent, institutional registry (https://clinicaltrials.gov/ Identifier: NCT03341377) and was first posted on 14 November 2017 but was actually started on 21 November 2017. Data for preliminary outcome measure were finally collected on 31 December 2019. Preregistration of this study involves registering the study design, variables, symptoms, and quality of life assessment instruments. In the above registration process, we did not preregister the analysis plan, because the amount of data involved in this study is huge and complex, and we can only select the appropriate analysis plan after preliminary analysis of the data, such as the mixed-effect models.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 1 was corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, X., Yu, H., Dai, W. et al. Patient-Reported Outcomes of Video-Assisted Thoracoscopic Surgery Versus Thoracotomy for Locally Advanced Lung Cancer: A Longitudinal Cohort Study. Ann Surg Oncol 28, 8358–8371 (2021). https://doi.org/10.1245/s10434-021-09981-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09981-1