Abstract
Background
Contrast esophagography often is performed to screen for anastomotic leakage (AL) after esophagectomy. However, its sensitivity remains low. Adverse events also have been reported. This report describes a new screening method to detect AL on computed tomography (CT) after esophagectomy.
Methods
From January 2012 to December 2015, 185 patients with esophageal cancer underwent surgical resection at the authors’ institution. The study comparatively reviewed patient characteristics, surgical outcomes, and findings from postoperative CT images and contrast esophagrams of 142 patients who underwent esophagectomy followed by primary gastric conduit reconstruction through a posterior mediastinum route.
Results
In this study, 24 patients (15.5%) had AL (leakage-positive group), and 120 patients (84.5%) did not (leakage-negative group). Both groups had comparable backgrounds. The number of air bubbles around the anastomotic site and the mediastinal space on postoperative CT images were significantly greater in the leakage-positive group than in the leakage-negative group. The cutoff value for the number of air bubbles required for a positive diagnosis of AL (“air bubble sign”) was calculated to be 3 by receiver operating characteristic curve. Compared with contrast esophagography, the air bubble sign on CT demonstrated a significantly higher sensitivity (86.4 vs. 50.0%) and an equivalent specificity (95.8 vs. 100.0%). Contrast esophagography altered the postoperative management of only five patients (3.5%).
Conclusions
A positive air bubble sign on CT is an objective and noninvasive screening method for AL after esophagectomy for esophageal cancer and may replace contrast esophagography as a screening test for AL.
Similar content being viewed by others
Esophageal cancer is the eighth most common cancer worldwide, affecting more than 450,000 people and having an increasing incidence.1 Primary therapy is selected according to tumor stage and location, histologic type, and the patient’s performance status and comorbidities.2 Surgical resection is the mainstay of treatment for a limited or locally advanced disease.2
Anastomotic leakage (AL) is a critical complication after esophagectomy. The frequency of AL reaches 35%,3,4,5,–6 and 50% of perioperative deaths are reported to occur because of AL.4
Several precautionary measures are attempted to prevent AL.7,8 However, the reported incidence remains high. Early diagnosis and treatment initiation are essential to minimize complications.
To date, contrast esophagography often has been used as a screening test for detecting occult AL.9,10,–11 However, in the absence of clinical signs and symptoms, the reported sensitivity of contrast esophagography for AL remains as low as 40–66%.9,11 The accuracy of this method strongly depends on the examiner’s skill. Furthermore, the widespread application of contrast esophagography may be associated with adverse events such as contrast aspiration and mediastinitis. Thus, the routine use of contrast esophagography has been questioned.9,10,11,–12
At our institution, we developed a new diagnostic procedure for AL after esophagectomy using computed tomography (CT) to enumerate air bubbles. In this study, we hypothesized that CT would be superior to contrast esophagography as a screening test for AL.
Methods
Patients
From January 2012 to December 2015, 185 patients with histologically diagnosed esophageal squamous cell carcinoma or adenocarcinoma underwent curative resection by transthoracic esophagectomy with extended lymph node (LN) dissection at our institution. We comparatively reviewed patient characteristics, surgical outcomes, and findings from postoperative CT images and contrast esophagrams of 142 patients who underwent esophagectomy followed by primary gastric conduit reconstruction through a posterior mediastinum route, which is the standard procedure at our institution. The extent of tumor spread was evaluated using the 7th edition of the tumor–node–metastasis (TNM) classification established by the Union for International Cancer Control.
On the basis of the Japan Clinical Oncology Group 9907 study, neoadjuvant chemotherapy, which combines cisplatin and 5-fluorouracil twice every 3 weeks,13 has been the standard treatment for clinical stages 2 and 3 patients at our institution. A regimen comprising three drugs (cisplatin, 5-fluorouracil, and docetaxel) administered thrice every 3 weeks is another treatment option.14 Locally recurrent cases and those with an incomplete response after definitive chemoradiotherapy (cisplatin and 5-fluorouracil with concurrent radiation of 50.4–60 Gy) were treated with salvage surgery and included in this study.
Endoscopic treatment was performed for superficial esophageal cancer (epithelium or lamina propria mucosae) without LN metastasis. However, patients with noncurative results underwent additional surgical resection and were also included in this study.
Surgical Procedure
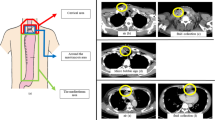
All operations were performed by three specialist esophageal cancer surgeons. Minimally invasive transthoracic esophagectomy and gastric conduit reconstruction with jejunostomy were performed as standard surgical procedures, as previously described.15 Patients for whom performing gastric conduit reconstruction was difficult because of synchronous double gastric cancer or a history of gastrectomy were excluded. Esophagectomy using a thoracoscope and laparoscope was the standard procedure. However, patients with T4 tumors or a history of thoracic surgery underwent open surgery. Two drainage tubes (at superior mediastinum and anastomotic sites) were inserted in the cervical region (Fig. 1), and one thoracic drainage tube was inserted on the dorsal side of the right thorax after reconstruction.
Gastric Conduit Formation and Anastomosis
After the transection of the abdominal esophagus, the stomach was exteriorized through the incisional wound. The stomach then was divided from the lesser curvature to the fornix using linear staplers. Next, the staple line of the gastric tube was covered by seromuscular suture. Pyloroplasty was performed using a finger fracture method. Esophagogastrostomy was performed in the left neck or thorax.
For patients with cervical anastomoses, the gastric conduit was pulled up to the left neck through the posterior mediastinal route. The cervical esophagus and gastric conduit then were anastomosed using a circular stapler. If the gastric conduit was not of sufficient length for mechanical anastomosis, the anastomosis was hand-sewn.
For patients with intrathoracic anastomoses, esophagogastrostomy was performed using a circular stapler through a minithoracotomy, as previously described.16 Nasogastric tubes were inserted during the surgery through the anastomotic site and positioned to lie in the gastric conduit 3 cm above the diaphragm after anastomosis.
LN Dissection
Mediastinal LNs with bilateral recurrent nerve LNs, pericardial LNs, and LNs along the lesser curvature and the left gastric artery were routinely dissected. If the primary tumor was located between the upper and middle thoracic esophagus, supraclavicular LNs also were dissected. The thoracic duct was routinely dissected to achieve further radical LN dissection. However, the thoracic duct was preserved in patients with liver cirrhosis, kidney disease, or heart disease.17
Perioperative Management
All the patients consumed an enteral diet of 400 ml/day orally for 5 days before the surgery. Hydrocortisone (200 mg/day) was administered for 5 days (i.e., 2 days before surgery to 3 days after surgery). Immediately after surgery, 24-h continuous jejunostomy feeding of an elemental diet was initiated as previously described.18
The patients were supported with an artificial ventilator in the intensive care unit for 24 h after surgery. The position of the nasogastric tube was monitored daily after surgery by chest radiography. Drainage tubes other than the tube for the anastomotic site were removed between postoperative days (PODs) 2 and 5 (when the drainage weight was less than 20 g/day).
The patients underwent thin-slice (slice thickness 1.25 mm) CT examination (Revolution CT; GE Healthcare, Chicago, IL, USA) on POD 6 and contrast esophagography on POD 7. On the basis of CT and contrast esophagography results, the nasogastric tube was removed for patients without AL basically on POD 7, with oral intake initiated on POD 8. The drainage tube for the anastomotic site was removed after peroral intake. Patients who recovered well were discharged after POD 14.
Air Bubble Sign on CT Images
Air densities around the anastomotic site and mediastinal space larger than 2 mm in the minor axis on axial CT images were considered to be air bubbles (Fig. 2a). Air densities continuous with skin incisions were considered to be subcutaneous emphysema and excluded. Low-density areas in contact with bones, drains, and surgical staplers were considered to be artifacts and also were excluded (Fig. 2b). The number of air bubbles was counted by two surgeons blinded from the study.
a Air bubbles in the cervical region and mediastinal space on the axial view of the computed tomography (CT) image (solid arrow). b Subcutaneous emphysema and air densities considered to be artifacts were excluded (dotted arrow). c Extraluminal contrast extravasation from the neoesophagus on contrast esophagography
Diagnosis of AL
Contrast esophagography was performed using non-ionic contrast agents. Examination and radiogram interpretation were performed by more than two skillful surgeons specializing in upper gastrointestinal surgery and familiar with contrast esophagography. Diagnosis of AL was based on clinical findings or observation of extraluminal contrast extravasation from the neoesophagus (Fig. 2c). In equivocal cases, AL was confirmed using CT or endoscopy. Gastric tube stump leakage and AL were not differentiated. The severity of complications was classified using the Clavien–Dindo (CD) classification of surgical complications.19 In this study, AL was defined as a leakage with a CD classification grade 2 or above. Cure of AL was comprehensively evaluated by clinical symptoms, blood tests, endoscopy, and imaging studies such as CT and drain-contrast radiography.
Data Analysis
The postoperative data collected included patient background data, surgical outcomes, and results of postoperative CT images and contrast esophagrams. The receiver operating characteristic (ROC) curve was used to predict the cutoff value for the number of air bubbles (“air bubble sign”). Sensitivity, specificity, positive predictive value, and negative predictive value were calculated for contrast esophagography and air bubble sign. The accuracies of contrast esophagraphy and air bubble sign on CT were compared using McNemar’s test for correlated proportions. For patients with AL, the date of diagnosis, AL diagnostic examination, CD classification grade, and duration of hospital stay were determined.
Statistical analyses comparing the groups were performed using the Mann–Whitney U test and the χ2 test with the IBM SPSS statistical software version 23 (IBM Corporation, Armonk, NY, USA). A p value lower than 0.05 was considered to be statistically significant. Unless otherwise indicated, data are presented as mean ± standard deviation. The study protocol was approved by the Ethics Committee of Keio University School of Medicine (Approval No. 20150044).
Results
Patient Background Data
From January 2012 to December 2015, 142 esophageal cancer patients underwent gastric conduit reconstruction through the posterior mediastinum route after transthoracic esophagectomy with extended LN dissection at our institution. Eight patients who underwent salvage surgery after definitive chemoradiotherapy also were included in the study. Of the 142 patients, 22 (15.5%) had AL (leakage-positive group), and 120 (84.5%) had no AL (leakage-negative group). The two groups did not differ significantly in terms of patient characteristics (Table 1).
Surgical Outcomes
In this study, 13 patients who underwent intrathoracic anastomosis had no AL, whereas AL developed in 22 (17.1%) of the 129 patients who underwent cervical anastomosis (p = 0.105). In addition, longer operative duration and open surgery were positively correlated with AL (p = 0.099 and 0.074, respectively). Other surgical factors were not correlated with AL (Table 1).
Contrast Esophagography Versus the Air Bubble Sign on CT for Diagnosing AL
After esophagectomy, patients generally underwent CT on POD 6 and contrast esophagography on POD 7. The exact schedule of examinations varied, depending on the patient’s condition and day of operation (Table 1). The 22 patients with AL had their diagnosis determined after their CT examination. For 11 of the 22 patients who experienced postoperative AL, extraluminal contrast extravasation on contrast esophagography was observed. The number of air bubbles around the anastomotic site and mediastinal space was significantly higher in the leakage-positive group than in the leakage-negative group (4.8 ± 2.3 vs. 0.7 ± 1.1; p < 0.001; Table 1).
The cutoff value of the number of air bubbles for an AL diagnosis was defined as 3 by the ROC curve using Yoden’s Index [area under the curve (AUC) 0.939; p < 0.001; Fig. 3]. Compared with contrast esophagography, the air bubble sign on CT demonstrated a significantly higher sensitivity (86.4 vs. 50.0%) and an equivalent specificity (95.8 vs. 100.0%; Table 2). The overall agreement of contrast esophagography and the air bubble sign on CT was 88%, with moderate correlation (κ coefficient 0.457, 95% confidence interval (CI) 0.350–0.564]. By McNemar’s test for correlated proportions, the sensitivity of the air bubble sign on CT was significantly higher than that of contrast esophagography for detecting AL after esophagectomy (p < 0.001).
Detailed Information of Patients Who Developed AL
Of the 22 patients who experienced AL, the diagnosis was determined for 5 patients using contrast esophagography, for 3 patients using CT imaging, and for 6 patients based on findings from both CT and contrast esophagography. Other patients had their AL diagnosis determined on the basis of clinical findings (seven patients) or endoscopic examination (one patient). One patient underwent endoscopic examination because the source for inflammation response was not detected by other measures. The patient who underwent endoscopy had one leakage site, at the anastomosis. Conservative measures improved the condition of 10 patients, whereas 12 patients required drainage or surgical intervention. For 10 of the 11 patients who did not show any abnormal findings by esophagography, a positive air bubble sign was observed on the postoperative CT image.
Discussion
Transthoracic esophagectomy is associated with a high frequency of postoperative complications such as pneumonia, recurrent laryngeal nerve paralysis, and AL.4,13 The overall morbidity reaches 43%.20,21 Postoperative contrast esophagography has often been routinely performed to assess neoesophageal function, in addition to detecting AL. Identifying the causes of swallowing dysfunction is important for patient treatment decisions, and in this regard, contrast esophagography plays an important role.22,23 However, the routine use of this screening test to detect occult AL after esophagectomy has been questioned given its low sensitivity and potential for complications.5,6,9,10,11,–12
In 2014, Cools-Lartigue et al.24 reported the results of postoperative barium esophagraphy, concluding that barium esophagraphy is not sufficiently sensitive to screen effectively for AL. The reported sensitivity of barium esophagraphy was 45.5% and resulted in a beneficial alteration to treatment for only 5 patients (2.5%), whereas it was misleading or harmful for 15 patients (7.4%). However, an ideal testing method for AL after esophagectomy remained to be established.
In this study, we developed a new diagnostic method (“air bubble sign” on CT). This method is a noninvasive and useful imaging method for complications such as pneumonia, atelectasis, deep surgical-site infection, and occult AL after esophagectomy. Because most of the postoperative deaths after esophagectomy are caused by pulmonary complications and AL,4,25 we performed CT on POD 6 as a screening test before peroral intake to minimize complications by early detection and initiation of treatment. Previously, AL has been diagnosed on CT images on the basis of considerable fluid collection or large air density around the anastomotic site. In our study, three of the patients without clinical findings or abnormal findings on contrast esophagram had their AL diagnosed according to such findings on CT images alone. However, for the majority of patients, AL develops in the absence of such findings. Instead, multiple small air bubbles around the anastomotic site and mediastinal space have been observed in patients with occult AL.
Enumerating air bubbles on CT is an easy and objective method, and the results do not vary among investigators, whereas the inspection accuracy of contrast esophagography strongly depends on the examiner’s skill. Accordingly, the current study analyzed the associations of AL and the number of air bubbles, and the inspection accuracy of contrast esophagography and the air bubble sign on CT for AL in order to develop a superior examination for AL after esophagectomy. Air bubbles were defined as air densities larger than 2 mm in the minor axis because 2 mm was the smallest detectable size by the thin-slice CT performed in our study.
The number of air bubbles was significantly higher in the patients with AL. To apply this finding as a diagnostic measure for AL, the cutoff value for a positive air bubble sign was calculated to be 3 using the ROC curve. False-positivity and false-negativity caused by subcutaneous emphysema, artifacts, surgical-site infection, and drainage tubes are inevitable. However, compared with contrast esophagography, the air bubble sign on CT demonstrated a significantly higher sensitivity (86.4 vs. 50.0%) and an equivalent specificity (95.8 vs. 100.0%) for detecting AL. The detection accuracy of contrast esophagography was comparable with those previously reported.9,10,24 However, only five patients (3.5%) had their management altered by the findings of contrast esophagography alone. Furthermore, 131 patients (92.3%) had a risk for contrast esophagography adverse effects, such as aspiration pneumonia, diarrhea, and vomiting, without any clinical benefit, as previously reported.9,10,11,–12,24
Using conventional measures, the mean time to diagnosis of AL after esophagectomy was 9.4 days. By introducing the air bubble sign as a diagnostic measure, AL would be diagnosed by POD 5.4 for the majority of affected patients. Furthermore, in the current study, 11 of 22 patients who experienced AL did not show any abnormal findings by esophagography. However, a positive air bubble sign was observed in the postoperative CT image for 10 of these patients. The air bubble sign would enable earlier initiation of treatment or strict observation, probably minimizing complications, shortening hospital stays, and reducing hospital expenses, thus outweighing the cost of CT. In addition, postoperative CT as a screening test leads to the early detection of other postoperative complications and may result in clinical and economic benefits.
This study had some limitations, particularly the retrospective nature of the analysis. A prospective study to determine the appropriate timing for performing CT is recommended. In addition, although this study contained a large number of patients who underwent esophagectomy for esophageal cancer, a relatively small number had postoperative AL, which may have affected the accuracy of the analysis.
Finally, although this study demonstrated the weaknesses of contrast esophagography as a screening test for postoperative AL, contrast esophagraphy still provides essential information regarding swallowing and neoesophageal function after esophagectomy. In this regard, contrast esophagography is a valuable method for selected patients.
Conclusions
The air bubble sign on CT is an objective and noninvasive screening method for AL after esophagectomy for esophageal cancer. In this study, the air bubble sign on CT had significantly higher sensitivity and equivalent specificity than contrast esophagography, and thus should be introduced to complement contrast esophagography.
References
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381:400–12.
Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D, Committee EG. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v50–7.
Page RD, Shackcloth MJ, Russell GN, Pennefather SH. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg. 2005;27:337–43.
Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N. Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001;88:1346–51.
Boone J, Rinkes IB, van Leeuwen M, van Hillegersberg R. Diagnostic value of routine aqueous contrast swallow examination after oesophagectomy for detecting leakage of the cervical oesophagogastric anastomosis. ANZ J Surg. 2008;78:784–90.
Honing J, Pultrum BB, van der Jagt EJ, Groen H, Plukker JT. Routine or on demand radiological contrast examination in the diagnosis of anastomotic leakage after esophagectomy. J Surg Oncol. 2009;100:699–702.
Nishi M, Hiramatsu Y, Hioki K, et al. Risk factors in relation to postoperative complications in patients undergoing esophagectomy or gastrectomy for cancer. Ann Surg. 1988;207:148–54.
Aiko S, Yoshizumi Y, Tsuwano S, Shimanouchi M, Sugiura Y, Maehara T. The effects of immediate enteral feeding with a formula containing high levels of omega-3 fatty acids in patients after surgery for esophageal cancer. J Parenter Enter Nutr. 2005;29:141–7.
Solomon DG, Sasaki CT, Salem RR. An evaluation of the routine use of contrast radiography as a screening test for cervical anastomotic integrity after esophagectomy. Am J Surg. 2012;203:467–71.
Swanson JO, Levine MS, Redfern RO, Rubesin SE. Usefulness of high-density barium for detection of leaks after esophagogastrectomy, total gastrectomy, and total laryngectomy. Am J Roentgenol. 2003;181:415–20.
Tirnaksiz MB, Deschamps C, Allen MS, Johnson DC, Pairolero PC. Effectiveness of screening aqueous contrast swallow in detecting clinically significant anastomotic leaks after esophagectomy. Eur Surg Res. 2005;37:123–8.
Gollub MJ, Bains MS. Barium sulfate: a new (old) contrast agent for diagnosis of postoperative esophageal leaks. Radiology 1997;202:360–2.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60.
Kaburagi T, Takeuchi H, Kawakubo H, Omori T, Ozawa S, Kitagawa Y. Clinical utility of a novel hybrid position combining the left lateral decubitus and prone positions during thoracoscopic esophagectomy. World J Surg. 2014;38:410–8.
Takeuchi H, Oyama T, Saikawa Y, Kitagawa Y. Novel thoracoscopic intrathoracic esophagogastric anastomosis technique for patients with esophageal cancer. J Laparoendosc Adv Surg Tech A 2012;22:88–92.
Matsuda S, Takeuchi H, Kawakubo H, et al. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine (Baltim) 2016;95:e3839.
Takesue T, Takeuchi H, Ogura M, et al. A prospective randomized trial of enteral nutrition after thoracoscopic esophagectomy for esophageal cancer. Ann Surg Oncol. 2015;22(Suppl 3):S802–9.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260:259–66.
Takeuchi H, Miyata H, Ozawa S, et al. Comparison of short-term outcomes between open and minimally invasive esophagectomy for esophageal cancer using a nationwide database in Japan. Ann Surg Oncol. 2017;24:1821–7.
Kato H, Miyazaki T, Sakai M, et al. Videofluoroscopic evaluation in oropharyngeal swallowing after radical esophagectomy with lymphadenectomy for esophageal cancer. Anticancer Res. 2007;27:4249–54.
Lanuti M, de Delva PE, Wright CD, et al. Post-esophagectomy gastric outlet obstruction: role of pyloromyotomy and management with endoscopic pyloric dilatation. Eur J Cardiothorac Surg. 2007;31:149–53.
Cools-Lartigue J, Andalib A, Abo-Alsaud A, et al. Routine contrast esophagram has minimal impact on the postoperative management of patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2014;21:2573–9.
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–32.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoji, Y., Takeuchi, H., Fukuda, K. et al. Air Bubble Sign: A New Screening Method for Anastomotic Leakage After Esophagectomy for Esophageal Cancer. Ann Surg Oncol 25, 1061–1068 (2018). https://doi.org/10.1245/s10434-017-6327-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6327-z