Abstract
Background
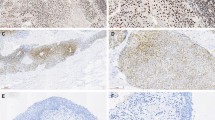
Inactivating mutation and consequent expression loss of stromal antigen 2 (STAG2, also known as SA2), a component of the cohesion complex, is one of the most common genetic aberrations in urothelial carcinoma. However, the clinicopathologic or prognostic significance of STAG2 alterations in upper tract urothelial carcinoma (UTUC) is largely unknown.
Methods
This study immunohistochemically examined the expression of STAG2 in 171 patients with UTUC. The correlations of STAG2 loss with clinicopathologic features and patients’ prognoses were examined.
Results
Loss of STAG2 expression was observed in 28 cases (16%). Loss of STAG2 was significantly correlated with histological low grade, papillary architecture, noninvasive tumors, absence of concomitant carcinoma in situ, and lower Ki-67 expression. Loss of STAG2 alone was not significantly associated with patients’ prognoses in either the uni- or multivariate analysis. However, STAG2 loss was significantly associated with worse clinical outcome in UTUC with high Ki-67 proliferation indexes, but not in UTUC with low Ki-67 expression.
Conclusions
Loss of STAG2 was generally associated with less aggressive features in UTUC. However, the STAG2 loss was an ominous sign in the subpopulation with higher Ki-67 proliferation indexes. Examining both STAG2 and Ki-67 status may be useful for identifying aggressive clinical behavior of UTUC.



Similar content being viewed by others
References
Balbas-Martinez C, Sagrera A, Carrillo-de-Santa-Pau E, et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy. Nat Genet. 2013;45:1464–9.
Guo G, Sun X, Chen C, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45:1459–63.
Solomon DA, Kim JS, Bondaruk J, et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat Genet. 2013;45:1428–30.
Kleyman M, Kabeche L, Compton DA. STAG2 promotes error correction in mitosis by regulating kinetochore-microtubule attachments. J Cell Sci. 2014;127:4225–33.
Kim MS, Kim SS, Je EM, Yoo NJ, Lee SH. Mutational and expressional analyses of STAG2 gene in solid cancers. Neoplasma. 2012;59:524–9.
Solomon DA, Kim T, Diaz-Martinez LA, et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science. 2011;333:1039–43.
Li X, Zhang TW, Tang JL, et al. Loss of STAG2 causes aneuploidy in normal human bladder cells. Genet Mol Res. 2015;14:2638–46.
Sfakianos JP, Cha EK, Iyer G, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015;68:970–7.
Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501.
Qiao Y, Zhu X, Li A, Yang S, Zhang J. Complete loss of STAG2 expression is an indicator of good prognosis in patients with bladder cancer. Tumour Biol. 2016;37:10279–86.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs–Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106–19.
Ichimura T, Morikawa T, Kawai T, et al. Prognostic significance of CD204-positive macrophages in upper urinary tract cancer. Ann Surg Oncol. 2014;21:2105–12.
Morikawa T, Maeda D, Kume H, Homma Y, Fukayama M. Ribonucleotide reductase M2 subunit is a novel diagnostic marker and a potential therapeutic target in bladder cancer. Histopathology. 2010;57:885–92.
Morikawa T, Sugiyama A, Kume H, et al. Identification of Toll-like receptor 3 as a potential therapeutic target in clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:5703–9.
Bailey ML, O’Neil NJ, van Pel DM, Solomon DA, Waldman T, Hieter P. Glioblastoma cells containing mutations in the cohesin component STAG2 are sensitive to PARP inhibition. Mol Cancer Ther. 2014;13:724–32.
Oosterhuis JW, Schapers RF, Janssen-Heijnen ML, Smeets AW, Pauwels RP. MIB-1 as a proliferative marker in transitional cell carcinoma of the bladder: clinical significance and comparison with other prognostic factors. Cancer. 2000;88:2598–605.
Wu TT, Chen JH, Lee YH, Huang JK. The role of bcl-2, p53, and ki-67 index in predicting tumor recurrence for low-grade superficial transitional cell bladder carcinoma. J Urol. 2000;163:758–60.
Curtin K, Slattery ML, Holubkov R, et al. p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2004;12:80–6.
Fromont G, Roupret M, Amira N, et al. Tissue microarry analysis of the prognostic value of E-cadherin, Ki67, p53, p27, survivin and MSH2 expression in upper urinary tract transitional cell carcinoma. Eur Urol. 2005;48:764–70.
Kim JS, He X, Orr B, et al. Intact cohesion, anaphase, and chromosome segregation in human cells harboring tumor-derived mutations in STAG2. PLoS Genet. 2016;12:e1005865.
Taylor CF, Platt FM, Hurst CD, Thygesen HH, Knowles MA. Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum Mol Genet. 2014;23:1964–74.
Fontana D, Bellina M, Gubetta L, et al. Monoclonal antibody Ki-67 in the study of the proliferative activity of bladder carcinoma. J Urol. 1992;148:1149–51.
Santos L, Amaro T, Costa C, et al. Ki-67 index enhances the prognostic accuracy of the urothelial superficial bladder carcinoma risk group classification. Int J Cancer. 2003;105:267–72.
Tian Y, Ma Z, Chen Z, et al. Clinicopathological and prognostic value of Ki-67 expression in bladder cancer: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0158891.
Amirghofran Z, Monabati A, Khezri A, Malek-Hosseini Z. Apoptosis in transitional cell carcinoma of bladder and its relation to proliferation and expression of p53 and bcl-2. Pathol Oncol Res. 2004;10:154–8.
Drosten M, Sum EY, Lechuga CG, et al. Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway. Proc Natl Acad Sci USA. 2014;111:15155–60.
Rey A, Lara PC, Redondo E, Valdes E, Apolinario R. Overexpression of p53 in transitional cell carcinoma of the renal pelvis and ureter. Relation to tumor proliferation and survival. Cancer. 1997;79:2178–85.
Tirode F, Surdez D, Ma X, et al. Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discov. 2014;4:1342–53.
Acknowledgment
We are grateful to Harumi Yamamura and Kei Sakuma for their excellent technical support.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2017_6097_MOESM1_ESM.tif
Supplementary Fig. 1. Kaplan–Meier curves of metastasis-free survival according to STAG2 status in p53 negative group (A) and p53 positive group (B) and cancer-specific survival in p53 negative group (C) and p53 positive group (D) in 171 upper tract urothelial carcinoma patients after nephroureterectomy. Supplementary material 1 (TIFF 2033 kb)
Rights and permissions
About this article
Cite this article
Miyakawa, J., Morikawa, T., Miyama, Y. et al. Loss of Stromal Antigen 2 (STAG2) Expression in Upper Urinary Tract Carcinoma: Differential Prognostic Effect According to the Ki-67 Proliferating Index. Ann Surg Oncol 24, 4059–4066 (2017). https://doi.org/10.1245/s10434-017-6097-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6097-7