Abstract
Background
The granulocyte-to-lymphocyte ratio (GLR) in the blood may be a useful marker of surgical stress (SS) following surgery for cancer. The aim of the present study was to compare the SS by measuring the GLR, and further study the value of the GLR in predicting postoperative infectious complications (ICs).
Methods
Data from 201 gastric cancer patients at Fujian Medical University Union Hospital who were enrolled in our prospective randomized controlled trial were extracted for this study. Blood samples to perioperatively measure the GLR were routinely taken. Additional external validation was performed using the dataset (n = 135) from Nanfang Hospital who were enrolled in the same trial.
Results
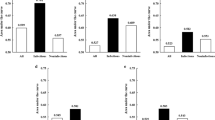
Patients undergoing either laparoscopic or open resection showed a similar preoperative GLR, as well as similar GLRs, on postoperative days (PODs) 1 and 3. The GLR on POD 5 was lower in the laparoscopic group compared with the open group (p = 0.014), and the optimal predictive GLR thresholds of postoperative ICs in both the laparoscopic and open groups were the same on POD 5, i.e. 6.5 and 7.4, respectively. The GLR on POD 5 was identified as an independent factor for postoperative ICs in both the laparoscopic and open groups. Similar results were found in the validation dataset.
Conclusion
The GLR status not only correlates with SS but may also be a reliable predictor of ICs for gastric cancer patients after gastrectomy. The ideal GLR thresholds on POD 5 for ICs following laparoscopic and open gastrectomy are 6.5 and 7.4, respectively.

Similar content being viewed by others
References
Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39–52.
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34:1350–7.
Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984;311:1413–8.
Perlmutter DH, Dinarello CA, Punsal PI, Colten HR. Cachctin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986;78:1349–54.
Meyer TA, Wang J, Tiao GM, Ogle CK, Fischer JE, Hasselgren PO. Sepsis and endotoxemia stimulate intestinal interleukin-6 production. Surgery. 1995;118:336–42.
Pritts T, Hungness E, Wang Q, Robb B, Hershko D, Hasselgren PO. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia role of transcription factors and regulation by stress response. Am J Surg. 2002;183:372–83.
Tabuchi T, Shimazaki J, Satani T, Nakachi T, Watanabe Y. The perioperative granulocyte/lymphocyte ratio is a clinically relevant marker of SS in patients with colorectal cancer. Cytokine. 2011;53:243–48
Sah BK, Chen MM, Yan M, Zhu ZG. Reoperation for early postoperative complications after gastric cancer surgery in a Chinese hospital. World J Gastroenterol. 2010;16:98–103.
Anonymous. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23
Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours. 7th ed. New York: Wiley-Liss; 2010.
Hayashi T, Yoshikawa T, Aoyama T, Hasegawa S, Yamada T, Tsuchida K, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer. 2015;18:368–74.
Artinyan A, Orcutt ST, Anaya DA, et al. Infectious postoperative complications decrease long-term survival in patients under going curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261:497–505.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Vulliamy P, McCluney S, Mukherjee S, Ashby L, Amalesh T. Postoperative elevation of the neutrophil: lymphocyte ratio predicts complications following esophageal resection. World J Surg. 2016; 40:1397–403.
Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258–65.
Okholm C, Goetze JP, Svendsen LB, Achiam MP. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol. 2014;49:1027–34.
Miyake H, Kawabata G, Gotoh A, Fujisawa M, Okada H, Arakawa S, et al. Comparison of surgical stress between laparoscopy and open surgery in the field of urology by measurement of humoral mediators. Int J Urol. 2002;9:329–33.
Jurczok A, Zacharias M, Wagner S, Hamza A, Fornara P. Prospective non-randomized evaluation of four mediators of the systemic response after extraperitoneal laparoscopic and open retropubic radical prostatectomy. BJU Int. 2007;99:1461–6.
Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, et al. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19:4168–77
Lloyd AR, Oppenheim JJ. Poly’s lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–72.
Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg. 2000;231:506–11.
Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–10.
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83.
Shishido Y, Fujitani K, Yamamoto K, Hirao M, Tsujinaka T, Sekimoto M. C-reactive protein on postoperative day 3 as a predictor of infectious complications following gastric cancer resection. Gastric Cancer. 2016;19:293–301.
Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EM, Noordzij PG. Postoperative interleukin-6 level and early detection of complications after elective major abdominal surgery. Ann Surg. 2016;263:1207–12.
Kim EY, Yim HW, Park CH, Song KY. C-reactive protein can be an early predictor of postoperative complications after gastrectomy for gastric cancer. Surg Endosc. 2017;31(1):445–54.
Disclosure
Jun Lu, Hao Liu, Long-long Cao, Chao-hui Zheng, Ping Li, Jian-wei Xie, Jia-bin Wang, Jian-xian Lin, Qi-yue Chen, Mi Lin, Ru-hong Tu, Guo-xin Li, and Chang-ming Huang declare no conflict of interest in connection with the publication of this work.
Funding
This work was supported by the National Key Clinical Specialty Discipline Construction Program of China (No. [2012] 649), the Key Projects of Science and Technology Plan of Fujian Province (No. 2014Y0025), and the Fund of Fujian Province science and technology innovation talents. Scientific and technological innovation joint capital projects of Fujian Province (2016Y9031).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, J., Liu, H., Cao, Ll. et al. The Granulocyte-to-Lymphocyte Ratio as a Marker of Surgical Stress and a Predictor of Postoperative Infectious Complications After Gastric Cancer Surgery: An Analysis of Patients Enrolled in a Prospective Randomized Trial. Ann Surg Oncol 24, 2688–2697 (2017). https://doi.org/10.1245/s10434-017-5846-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5846-y