Abstract
Purpose
To identify a tolerable and effective dose for 5-fraction stereotactic body radiotherapy for hepatic metastases.
Methods
Patients were enrolled onto three dose-escalation cohorts: 30 Gy in 3 fractions, 50 Gy in 5 fractions, and 60 Gy in 5 fractions. Eligible patients had one to five hepatic metastases, ability to spare a critical hepatic volume (volume receiving <21 Gy) of 700 ml, adequate baseline hepatic function, no concurrent antineoplastic therapy, and a Karnofsky performance score of ≥60. Dose-limiting toxicity included treatment-related grade 3 toxicity in the gastrointestinal, hepatobiliary/pancreas, and metabolic/laboratory categories. Any grade 4 or 5 event attributable to therapy was defined as a dose-limiting toxicity. Local control (LC) and complete plus partial response rates were assessed.
Results
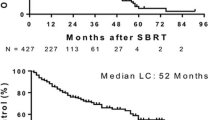
Twenty-seven patients, 9 in each cohort, with 37 lesions were enrolled and treated: 17 men and 11 women; median age 62 (range 48–86) years; most common site of primary disease, colorectal (44.4%). Median follow-up was 20 (range 4–53) months. There was no grade 4 or 5 toxicity or treatment-related grade 3 toxicity. Actuarial 24-month LC rates for the 30-, 50-, and 60-Gy cohorts were 56%, 89%, and 100%, respectively. There was a statistically significant difference for LC between the 60- and 30-Gy cohorts (P = 0.009) but not between the 60- and 50-Gy cohorts (P = 0.56) or the 50- and 30-Gy cohorts (P = 0.091). The maximum tolerated dose was not reached.
Conclusions
A dose of 60 Gy in 5 fractions can be safely delivered to selected patients with hepatic metastases as long as the critical liver volume is respected. A dose of 60 Gy in 5 fractions yields an excellent level of LC.


Similar content being viewed by others
References
Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25.
Fong Y, Blumgart LH, Cohen AM. Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin. 1995;45:50–62.
Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18.
Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59–71.
Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66.
Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–77.
Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–22.
de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–25.
Giovannini M, Seitz JF. Ultrasound-guided percutaneous alcohol injection of small liver metastases. Results in 40 patients. Cancer. 1994;73:294–7.
Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361:368–73.
Shibata T, Niinobu T, Ogata N, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276–84.
Sotsky TK, Ravikumar TS. Cryotherapy in the treatment of liver metastases from colorectal cancer. Semin Oncol. 2002;29:183–91.
Tandan VR, Harmantas A, Gallinger S. Long-term survival after hepatic cryosurgery versus surgical resection for metastatic colorectal carcinoma: a critical review of the literature. Can J Surg. 1997;40:175–81.
Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–66.
Vogl TJ, Muller PK, Hammerstingl R, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995;196:257–65.
Vogl TJ, Straub R, Eichler K, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology. 2002;225:367–77.
Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–70.
Blomgren H, Lax I, Goranson H, et al. Radiosurgery for tumors in the body: clinical experience using a new method. J Radiosurgery. 1998;1:63–74.
Herfarth KK, Debus J. [Stereotactic radiation therapy for liver metastases]. Chirurg. 2005;76:564–9.
Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164–70.
Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–47.
Wulf J, Hadinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol. 2001;177:645–55.
Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–8.
Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–8.
Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–8.
Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–8.
Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–52.
Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I–II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 200973:1235–42.
Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–22.
Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Mulier S, Ni Y, Jamart J, et al. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:144–57.
Gravante G, Sconocchia G, Ong SL, et al. Immunoregulatory effects of liver ablation therapies for the treatment of primary and metastatic liver malignancies. Liver Int. 2009;29:18–24.
Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6.
Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–52.
Herfarth KK, Debus J, Wannenmacher M. Stereotactic radiation therapy of liver metastases: update of the initial phase-I/II trial. Front Radiat Ther Oncol. 2004;38:100–5.
Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–55.
Wolbarst AB. Optimization of radiation therapy II: the critical-voxel model. Int J Radiat Oncol Biol Phys. 1984;10:741–5.
Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–21.
Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–55.
Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9.
Acknowledgment
Supported in part by NIH CTSA grant UL1 RR024982. R.D.T. received research grants from Varian Medical Systems, Palo Alto, California, and Accuray Inc., Sunnyvale, California (both manufacturers of stereotactic radiation equipment).
Conflict of interest
The other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rule, W., Timmerman, R., Tong, L. et al. Phase I Dose-Escalation Study of Stereotactic Body Radiotherapy in Patients With Hepatic Metastases. Ann Surg Oncol 18, 1081–1087 (2011). https://doi.org/10.1245/s10434-010-1405-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1405-5