Abstract
Background
Sentinel lymph node biopsy (SLNB) without axillary lymph node dissection (ALND) in SLN negative patients is a standard of care for most breast cancer patients. SLNB for axillary staging after primary systemic therapy (PST) is still under discussion because of possibly reduced accuracy, while data are lacking. The purpose of this study was to evaluate the accuracy of SLNB after PST.
Materials and Methods
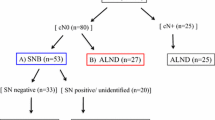
A total of 185 breast cancer patients were treated with PST; 160 patients received preoperative chemotherapy, and 25 patients received preoperative endocrine therapy. Thus, 143 of 160 patients with preoperative chemotherapy and 22 of 25 patients with preoperative endocrine therapy were eligible for evaluation. The combination of blue dye and radioactive tracer was used for identification of SLNs. All patients received SLNB and axillary lymph node dissection (ALND). Pathologic assessment of SLNs was performed and compared to non-SLN status.
Results
Pathologic complete response rates and breast conserving therapy rates were 15.4 and 78.3% in the preoperative chemotherapy group and 0 and 77.3% in the preoperative endocrine therapy group, respectively. Identification rate, sensitivity, overall accuracy, and false-negative rate were 81.1% (116 of 143), 91.7% (55 of 60), 95.7% (111 of 116), and 8.3% (5 of 60) in the preoperative chemotherapy group and 77.3% (17 of 22), 90.0% (9 of 10), 94.1% (16 of 17), and 10.0% (1 of 10) in the preoperative endocrine therapy group, respectively.
Discussion
SLNB after primary systemic therapy is accurate, and the results are comparable to those of primary SLNB. SLNB after PST could spare ALND in up to 40% of patients with primary positive axillary lymph nodes and should be considered as a standard for axillary staging in those patients.
Similar content being viewed by others
References
Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609.
Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS Trial. Ann Surg Oncol. 2010;17:1854–61.
Miltenburg DM, Miller C, Karamlou TB, Brunicardi FC. Meta-analysis of sentinel lymph node biopsy in breast cancer. J Surg Res. 1999;84:138–42.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8.
Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, Galimberti V, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600.
Van der Ploeg IM, Nieweg OE, van Rijk MC, Valdes Olmos RA, Kroon BB. Axillary recurrence after a tumour-negative sentinel node biopsy in breast cancer patients: a systematic review and meta-analysis of the literature. Eur J Surg Oncol. 2008;34:1277–84.
Kuerer HM, Hunt KK. The rationale for integration of lymphatic mapping and sentinel node biopsy in the management of breast cancer patients receiving neoadjuvant chemotherapy. Semin Breast Dis. 2002;5:80–7.
Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol. 1996;9:893–900.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
Von Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Capecitabine in addition to anthracyclin- and taxan-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol. 2010;28:2015–23.
Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–33.
Reitsamer R, Peintinger F, Rettenbacher L, Prokop E. Sentinel lymph node biopsy in breast cancer patients after neoadjuvant chemotherapy. J Surg Oncol. 2003;84:63–7.
Tausch C, Konstantiniuk P, Kugler F, Reitsamer R, Roka S, Pöstlberger S, et al. Sentinel lymph node biopsy after preoperative chemotherapy for breast cancer: findings from the Austrian Sentinel Node Study Group. Ann Surg Oncol. 2008;15:3378–83.
Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–702.
Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539–46.
Van Deurzen CH, Vriens BE, Tjan-Heijnen VC, van der Wall E, Albregts M, van Hilligersberg R, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: A systematic review. Eur J Cancer. 2009;45:3124–30.
Classe JM, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726–32.
Hunt KK, Yi M, Mittendorf EA, Guerrero C, Babiera GV, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009 Aug 27 [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reitsamer, R., Menzel, C., Glueck, S. et al. Sentinel Lymph Node Biopsy is Precise After Primary Systemic Therapy in Stage II–III Breast Cancer Patients. Ann Surg Oncol 17 (Suppl 3), 286–290 (2010). https://doi.org/10.1245/s10434-010-1246-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1246-2