Abstract
Background
IMP3 (insulinlike growth factor II mRNA-binding protein 3) is a newly identified oncofetal RNA-binding protein that is involved in cell growth and cell migration during the early stages of embryogenesis. This study sought to elucidate its role in tumor progression and prognosis of colorectal adenocarcinoma (CRA).
Methods
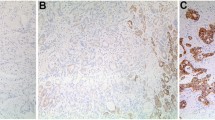
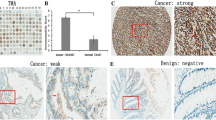
IMP3 expression in 186 surgically resected unifocal primary CRAs was analyzed by immunohistochemistry. The proportions of tumor cells positive for IMP3 were scored into diffuse (≥50%), focal or heterogeneous (10–50%), and trace (<10%), and the expression levels were correlated with clinicopathologic features and patient survival.
Results
Cytoplasmic immunoreactivity for IMP3 was diffuse in 66 (35%), focal or heterogeneous in 38 (21%), and trace in 34 (18%) samples. No staining was seen in the adjacent nontumorous tissue. Diffuse IMP3 expression correlated with large tumor (>3 cm, P = .0452), high-stage tumor (IIIa–IV, P = .0417), lymph node metastasis (P = .0232), high lymph node ratio (LNR ≥ .7, P = .0016), and lower 5-year survival (P = .0012). Further analysis showed that patients with high-stage CRA and diffuse IMP3 expression had the worst survival rate (P < .0001)—far worse than those without diffuse IMP3 expression (P = .0038). Moreover, multivariant analysis showed diffuse IMP3 expression, serosal invasion, LNR, tumor stage, and adjuvant chemotherapy were independent prognostic factors in CRA.
Conclusion
Diffuse IMP3 protein expression correlates with invasion and aggressiveness during cancer growth and metastasis, and it is an important prognostic factor of CRAs.




Similar content being viewed by others
References
Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61:561–9.
Liang JT, Cheng YM, Chang KJ, Chien CT, Hsu HC. Reappraisal of K-ras and p53 gene mutations in the recurrence of Dukes’ B2 rectal cancer after curative resection. Hepatogastroenterology. 1999;46:830–7.
Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6.
Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8.
Wang T, Fan L, Watanabe Y, et al. L523S, an RNAbinding protein as a potential therapeutic target for lung cancer. Br J Cancer. 2003;88:887–94.
Yantiss RK, Woda BA, Fanger GR, et al. KOC (Komology Domain Containing Protein Overexpressed in Cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol. 2005;29:188–95.
Nielsen J, Christiansen J, Lykke-Andersen J, et al. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–70.
Mueller-Pillasch F, Pohl B, Wilda M, et al. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999;88:95–9.
Li C, Rock KL, Woda BA, et al. IMP3 is a novel biomarker for adenocarcinoma in situ of the uterine cervix: an immunohistochemical study in comparison with p16(INK4a) expression. Mod Pathol. 2007;20:242–7.
Gress TM, Muller-Pillasch F, Geng M, et al. A pancreatic cancer-specific expression profile. Oncogene. 1996;13:1819–930.
Wang T, Hopkins D, Schmidt C, et al. Identification of genes differentially over-expressed in lung squamous cell carcinoma using combination of cDNA subtraction and microarray analysis. Oncogene. 2000;19:1519–28.
Jiang Z, Chu P, Woda BA, et al. Analysis of RNA binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7:556–64.
Hoffmann NE, Sheinin Y, Lohse CM, et al. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer. 2008;112:1471–9.
Zheng W, Yi X, Fadare O, et al. The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol. 2008;32:304–15.
Sitnikova L, Mendese G, Liu Q, et al. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res. 2008;14:1701–6.
Jeng YM, Chang CC, Hu FC, et al. Expression of RNA-binding protein IMP-3 promotes tumor invasion and predicts high tumor stage, early tumor recurrence, and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48:1118–27.
Mueller-Pillasch F, Lacher U, Wallrapp C, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729–33.
Liao B, Hu Y, Herrick DJ, Brewer G. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem. 2005;280:18517–24.
O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5.
Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–8.
Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag, 2002.
Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–96.
Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–63.
Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–9.
Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–12.
Telian SH, Bilchik AJ. Significance of the lymph node ratio in stage III colon cancer. Ann Surg Oncol. 2008;15:1557–8.
Megale Costa LJ, Soares HP, Gaspar HA, et al. Ratio between positive lymph nodes and total dissected axillaries lymph nodes as an independent prognostic factor for disease-free survival in patients with breast cancer. Am J Clin Oncol. 2004;27:304–6.
Woodward WA, Vinh-Hung V, Ueno NT, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–6.
Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61.
Inoue K, Nakane Y, Iiyama H, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27–34.
Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–74.
Schumacher P, Dineen S, Barnett C Jr., Fleming JTA. The metastatic lymph node ratio predicts survival in colon cancer. Am J Surg Pathol. 2007;194:827–31.
Lee HY, Choi HJ, Park KJ, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–7.
Jeng YM, Wang TH, Lu SH, Yuan RH, Hsu HC. Prognostic significance of insulin-like growth factor II mRNA-binding protein 3 expression in gastric adenocarcinoma. Br J Surg. 2008;96:66–73.
De Ridder M, Vinh-Hung V, Van Nieuwenhove Y, et al. Prognostic value of the lymph node ratio in node positive colon cancer. Gut. 2006;55:1681.
Vikesaa J, Hansen TV, Jonson L, et al. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J. 2006;25:1456–68.
Yaniv K, Fainsod A, Kalcheim C, Yisraeli JK. The RNA-binding protein Vg1 RBP is required for cell migration during early neural development. Development. 2003;130:5649–61.
Acknowledgment
This work was supported by a grant from the Tao-Yuan General Hospital, Department of Health, Executive Yuan, R.O.C. (PTH9701 to R.H.Y.). We thank Hong-I Hsu for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, RH., Wang, CC., Chou, CC. et al. Diffuse Expression of RNA-Binding Protein IMP3 Predicts High-Stage Lymph Node Metastasis and Poor Prognosis in Colorectal Adenocarcinoma. Ann Surg Oncol 16, 1711–1719 (2009). https://doi.org/10.1245/s10434-009-0446-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0446-0