Abstract
Background
Soluble intercellular adhesion molecule-1 (sICAM-1) represents a circulating form of ICAM-1 that is constitutively expressed or is inducible, which localizes to the cell surfaces of different cell lines and is related to the metastatic potential of cancer cells. The aim of the present study was to determine the relationships between the preoperative serum concentration of sICAM-1 and clinicopathological features, established tumor markers and prognosis, in colorectal cancer patients.
Methods
One hundred and thirty-eight patients with histologically proven colorectal cancer and 40 normal volunteers were included in this trial. Preoperative serum was collected, and sICAM-1 levels were assayed using a commercially available enzyme-linked immunosorbent assay kit.
Results
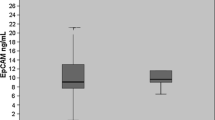
The mean sICAM-1 level in patients was significantly higher than that in controls, and increased with disease progression. The prognosis of patients with an elevated sICAM-1 level was significantly worse than that of patients with a normal sICAM-1 level. In a Cox multivariate analysis, the strongest prognostic factor in all patients was distant metastasis followed by sICAM-1 level, while in patients with stage II classification, the strongest prognostic factor was serum level of sICAM-1. The prognosis of stage II patients positive for sICAM-1 was comparable to that of stage III patients.
Conclusions
Preoperative sICAM-1 level is an independent prognostic marker for stage II colorectal cancer. Measuring serum sICAM-1 may provide valuable information, especially for stage II patients, when selecting appropriate candidates for adjuvant chemotherapy.





Similar content being viewed by others
References
Haller DG. An overview of adjuvant therapy for colorectal cancer. Eur J Cancer 1995; 31A:1255–63
Wein A, Hahn EG, Merkel S, et al. Adjuvant chemotherapy for stage II colon cancer. Eur J Surg Oncol 2000; 26:730–2
Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem 2001; 47:624–30
Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinoma by immunological tolerance and absorption techniques. J Exp Med 1965; 121:439–62
Moertel CG, Fleming TR, Macdonald JS, et al. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 1993; 270:943–7
Kimura Y, Fujieda S, Takabayashi T, et al. Conventional tumor markers are prognostic indicators in patients with head and neck squamous cell carcinoma. Cancer Lett 2000; 155:163–8
Smith ME, Thomas JA. Cellular expression of lymphocyte function associated antigens and the intercellular adhesion molecule-1 in normal tissue. J Clin Pathol 1990; 43:893–900
Rothlein R, Mainolfi EA, Czajkowski M, et al. A form of circulating ICAM-1 in human serum. J Immunol 1991; 147:3788–93
Banks RE, Gearing AJ, Hemingway IK, et al. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer 1993; 68:122–4
Velikova G, Banks RE, Gearing A, et al. Serum concentration of soluble adhesion molecules in patients with colorectal cancer. Br J Cancer 1998; 77:1857–63
Giavazzi R, Chirivi RG, Garofalo A, et al. Soluble intercellular adhesion molecule-1 is released by human melanoma cells and is associated with tumor growth in nude mice. Cancer Res 1992; 52:2628
Viac J, Vincent C, Palacio S, et al. Tumour necrosis factor (TNF) soluble receptors in malignant melanoma: correlation with soluble ICAM-1 levels. Eur J Cancer 1996; 32A:447
Alexiou D, Karayiannakis AJ, Syrigos KN, et al. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer 2001; 37:2392
Liu YZ, Chen B, She XD. A clinical evaluation of serum concentrations of intercellular adhesion molecule-1 in patients with gastric cancer. World J Gastroenterol 1998; 4:225
Nakata B, Hori T, Sunami T, et al. Clinical significance of serum soluble intercellular adhesion molecule 1 in gastric cancer. Clin Cancer Res 2000; 6:1175
Zhang GJ, Adachi I. Serum levels of soluble intercellular adhesion molecule-1 and E-selectin in metastatic breast carcinoma: correlations with clinicopathological features and prognosis. Int J Oncol 1999; 14:71
Duffy MJ. CEA as a marker for colorectal cancer: is it clinically useful. Clin Chem 2001; 47:624–30
Aster VB, Coller FA. The prognosis significance of direct extension of carcinoma of the colon and rectum. Ann Surg 1954; 192:846–50
Franzke A, Probst-Kepper M, Buer J, et al. Elevated pretreatment serum levels of soluble vascular cell adhesion molecule 1 and lactate dehydrogenase as predictors of survival in cutaneous metastatic malignant melanoma. Br J Cancer 1998; 78:40–5
Becker JC, Dummer R, Hartmann AA, et al. Shedding of ICAM-1 from human melanoma cell lines induced by INF-gamma and TNF-alpha: functional consequences on cell-mediated cytotoxicity. J Immunol 1991; 147:4398
Rokhlin OW, Cohen MB. Soluble forms of CD44 and CD54 (ICAM-1) cellular adhesion molecules are released by human prostatic cancer cell lines. Cancer Lett 1996; 107:29
Araki T, Miki C, Kusunoki M. Biological implications of circulating soluble intercellular adhesion molecule-1 in colorectal cancer patients. Scand J Gastroenterol 2001; 36:399–404
Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw 2004; 15:91–8
Makgoba MW, Sanders ME, Ginther Luce GE, et al. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol 1988; 18:637–40
Chong AS, Boussy IA, Jiang XL, et al. CD54/ ICAM-1 is a costimulator of NK cell-mediated cytotoxicity. Cell Immunol 1994; 157:92–105
Kamezaki S, Kurozawa Y, Iwai N, et al. Serum levels of soluble ICAM-1 and VCAM-1 predict pre-clinical cancer. Eur J Cancer 2005; 41:2355–9
Nakata B, Hori T, Sunami T, et al. Clinical significance of serum soluble intercellular adhesion molecule 1 in gastric cancer. Clin Cancer Res 2000; 6:1175–9
Mulcahy HE, Duffy MJ, Gibbons D, et al. Urokinase-type plasminogen activator andoutcome in Dukes’ B colorectal cancer. Lancet 1994; 344:583–84
Kay EW, Mulcahy H, Walsh CB, et al. Cytoplasmic c-erbB-2 protein expression correlates with survival in Dukes’ B colorectal carcinoma. Histopathology 1994; 25:455–61
Takahashi Y, Tucker SL, Kitadai Y, et al. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg 1997; 132:541–46
Dorudi S, Hanby AM, Poulsom R, et al. Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer 1995; 71:614–6
Graziano F, Cascinu S. Prognostic molecular markers for planning adjuvant chemotherapy trials in Dukes’ B colorectal cancer patients: how much evidence is enough? Ann Oncol 2003; 14:1026–38
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toiyama, Y., Miki, C., Inoue, Y. et al. Soluble Intercellular Adhesion Molecule-1 as a Prognostic Marker for Stage II Colorectal Cancer Patients. Ann Surg Oncol 15, 1617–1624 (2008). https://doi.org/10.1245/s10434-008-9874-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-9874-5