Abstract
Background
Invasive cell carcinoma of the bladder often develops after complete transurethral excision of superficial transitional cell carcinoma. It has been postulated that primary tumors release angiogenesis-blocking proteins which suppress distant metastases. We have identified an endogenous protein which might be responsible for tumor dormancy.
Methods
A transitional cell carcinoma cell line was developed (UMUC-3i) which inhibits the growth of a tumor implant at a distant site in SCID mice. Conditioned media of UMUC-3i cultured cells was first pooled and then fractioned, and the capacity of individual components to block endothelial cell growth was tested. The protein fraction responsible for blocking endothelial cell growth was identified by N-terminal amino acid sequencing as well as by mass-spectrometry. The effects of the purified protein in preventing endothelial cell proliferation and tube formation in an in vitro angiogenesis assay was investigated.
Results
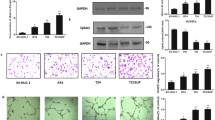
The plasma protein β2-glycoprotein-I (β2gpI) was isolated and identified from conditioned medium of UMUC-3i cultured cells. Based on the in vitro angiogenesis assay, β2gpI strongly inhibited endothelial cell growth and tube formation, whereby the inhibitory activity corresponded to the clipped version of β2gpI (cβ2gpI). Clipping was induced by adding plasmin at a molar ratio 1:15 (plasmin:substrate). Further analysis indicated that cβ2gpI effects were mediated by annexin II surface receptors expressed on endothelial cells.
Conclusions
cβ2gpI may be involved in blocking angiogenic processes and bladder cancer progression. In this case, cβ2gpI may be a promising tool in bladder cancer therapy.







Similar content being viewed by others
Abbreviations
- β2gpI::
-
β2glycoprotein-I
- cβ2gpI::
-
clipped version of β2gpI
- TCC::
-
Transitional cell carcinoma
References
Stein JP, Grossfeld GD, Ginsberg DA, Esrig D, Freeman JA, Figueroa AJ, Skinner DG, Cote RJ. Prognostic markers in bladder cancer: A contemporary review of the literature. J Urol 1998;160:645–59
Oosterlinck W, Lobel B, Jakse G, Malmstrom PU, Stockle M, Sternberg C. Guidelines on bladder cancer. Eur Urol 2002;41105–112
Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med 2003;3:643–51
O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses MA, Lane WS, Cao Y, Sage BH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994;79:315–28
O’Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science 1999;285:1926–8
Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995;1:149–53
Beecken W-D, Fernandez A, Joussen AM, Achilles E-G, Flynn E, Lo K-M, Gillies SD, Javaherian K, Folkman J, Shing Y. Effect of antiangiogenic therapy on slowly growing, poorly vascularized tumors in mice. J Natl Cancer Inst 2001;93:382–87
Beecken W-D, Fernandez A, Panigrahy D, Achilles E-G, Kisker O, Flynn E, Joussen AM, Folkman J, Shing Y. Efficacy of antiangiogenic therapy with TNP-470 in superficial and invasive bladder cancer models in mice. Urology 2000;56:521–26
Day JR, O’Hara PJ, Grant FJ, Lofton-Day C, Berkaw MN, Werner P, Arnaud P. Molecular cloning and sequence analysis of the cDNA encoding human apolipoproteinH (ß2-Glykoprotein-1). Int J Clin Lab Res 1992;21:256–63
Schultze HE, Heide K, Haupt H. Über ein bisher unbekanntes niedermolekulares ß2-Globulin des Humanserums. Naturwissenschaften 1961;148:719
Shi T, Iverson GM, Qi JC, Cockerill KA, Linnik MD, Konecny P, Krilis S. ß2-Glykoprotein-1 binds factor XI and inhibits its activation by thrombin and factor XIIa: Loss of inhibition by clipped ß2-glykoprotein-1. Proc Natl Acad Sci USA 2004;101:3939–943
Sheng Y, Sali A, Herzog H, Lahnstein J, Krilis SA. Site-directed mutagenesis of recombinant human beta 2-glycoprotein I identifies a cluster of lysine residues that are critical for phospholipid binding and anti-cardiolipin antibody activity. J Immunol 1996;157:744–51
Ohkura N, Hagihara Y, Yoshimura T, Goto Y, Kato H. Plasmin can reduce the function of human beta2 glycoprotein I by cleaving domain V into a nicked form. Blood 1998;91:4173–79
Horbach DA, van Oort ET, Lisman T, Maijers JC, Derksen RH, de Groot PG. Beta2-glycoprotein I is proteolytically cleaved in vivo upon activation of fibrinolysis. Thromb Haemostasis 1999;81:87–95
Itoh Y, Inuzuka K, Kohno I, Wada H, Shiku H, Ohkura N, Kato H. Highly increased plasma concentrations of the nicked form of beta(2) glycoprotein I in patients with leukemia and with lupus anticoagulant: measurement with a monoclonal antibody specific for a nicked form of domain V. J Biochem (Tokyo) 2000;128:1017–24
Nakaya Y, Schaefer EJ, Brewer HB Jr. Activation of human post heparin lipoprotein lipase by apolipoprotein H (beta 2-glycoprotein I). Biochem Biophys Res Commun 1980;95:1168–72
Shi W, Chong BH, Hogg PJ, Chesterman CN. Anticardiolipin antibodies block the inhibition by beta 2-glycoprotein I of the factor Xa generating activity of platelets. Thromb Haemostasis 1993;70:342–5
Nimpf J, Bevers EM, Bomans PH, Till U, Wurm H, Kostner GM, Zwaal RF. Prothrombinase activity of human platelets is inhibited by beta 2-glycoprotein-I. Biochim Biophys Acta 1986;884:142–149
Nimpf J, Wurm H, Kostner GM. Beta 2-glycoprotein-I (apo-H) inhibits the release reaction of human platelets during ADP-induced aggregation. Atherosclerosis 1987;63:109–14
Manfredi AA, Rovere P, Heltai S, Galati G, Nebbia G, Tincani A, Balestrieri G, Sabbadini MG. Apoptotic cell clearance in systemic lupus erythematosus. II. Role of beta2-glycoprotein I. Arthritis Rheum 1998;41:215–23
McNeil HP, Simpson RJ, Chersterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci USA 1990;87:4120–4
Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJ, Barbui T, Zwaal RF, Bevers EM. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet 1990;335:1544–7
Shevchenko A, Chernushevich I, Wilm M, Mann M. De Novo peptide sequencing by nanoelectrospray tandem mass spectrometry using triple quadrupole and quadrupole/time-of-flight instruments. Methods Mol Biol 2000;146:1–16
Guerin J, Sheng Y, Reddel S, Iverson GM, Chapman MG, Krilis SA. Heparin inhibits the binding of beta 2-glycoprotein I to phospholipids and promotes the plasmin-mediated inactivation of this blood protein. Elucidation of the consequences of the two biological events in patients with the anti-phospholipid syndrome. J Biol Chem 2002;277:2644–9
Michaelis M, Michaelis UR, Fleming I, Suhan T, Cinatl J, Blaheta RA, Hoffmann K, Kotchetkov R, Busse R, Nau H, Cinatl J Jr. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol 2004;65:520–7
Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem 2000;275:1521–4
Wurm H. beta 2-Glycoprotein-I (apolipoprotein H) interactions with phospholipid vesicles. Int J Biochem 1984;16:511–5
Polz E, Kostner GM. Binding of beta 2-glycoprotein-I to intralipid: determination of the dissociation constant. Biochem Biophys Res Commun 1979;90:1305–12
Ma K, Simantow R, Zhang J-C, Silverstein R, Hajjar KA, McCrae KR. High affinity binding of β2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem 2000;275:15541–48
Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 2005;105:1964–69
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31
O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996;2:689–92
Rübben H, Otto T. Lokal fortgeschrittenes oder metastasiertes Harnblasenkarzinom. Aktuelle Aspekte in der Therapie. Urologe (A) 2001;40:464–67
Westphal JR, Van’t Hullenaar R, Geurts-Moespot A, Sweep FC, Verheijen JH, Bussemakers MM, Askaa J, Clemmensen I, Eggermont AA, Ruiter DJ, De Waal RM. Angiostatin generation by human tumor cell lines: involvement of plasminogen activators. Int J Cancer 2000;86:760–67
Campbell SC, Volpert OV, Ivanovich M, Bouck NP. Molecular mediators of angiogenesis in bladder cancer. Cancer Res 1998;58:1298–304
Cai G, Satoh T, Hoshi H. Purification and characterization of an endothelial cell-viability maintaining factor from fetal bovine serum. Biochim Biophys Acta 1995;1269:13–8
Caronti B, Calderaro C, Alessandri C, Conti F, Tinghino R, Palladini G, Valesini G. Beta2-glycoprotein I (beta2-GPI) mRNA is expressed by several cell types involved in anti-phospholipid syndrome-related tissue damage. Clin Exp Immunol 1999;115:214–9
Knudsen BS, Silverstein RL, Leung LL, Harpel PC, Nachman RL. Binding of plasminogen to extracellular matrix. J Biol Chem 1986;261:10765–71
Li Z, Krilis SA. Anti-beta(2)-glycoprotein I antibodies and the antiphospholipid syndrome. Autoimmun Rev 2003;2:229–34
Wu HL, Chang BI, Wu DH, Chang LC, Gong CC, Lou KL, Shi GY. Interaction of plasminogen and fibrin in plasminogen activation. J Biol Chem 1990;265:19658–64
Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol 2001;106:33–42
Tsihlias J, Grossman HB. The utility of fibrin/fibrinogen degradation products in superficial bladder cancer. Urol Clin North Am 2000;27:39–46
Morgan RO, Fernandez MP. Annexin gene structures molecular evolutionary genetics. Cell Mol Life Sci 1997;53:508–15
Evans TC, Nelsestuen GL. Calcium and membrane-binding properties of monomeric and multimeric annexin II. Biochemistry 1994;33:13231–38
Emans N, Gorvel JP, Walter C, Gerke V, Kellner R, Griffiths G, Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol 1993;120:1357–69
Creutz CE. The annexins and exocytosis. Science 1992;258:924–31
Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-c. J Cell Biol 1994;126:539–48
Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator I. Identity with annexin II. J Biol Chem 1994;269:21191–97
Tokes AM, Hortovanyi E, Kulka J, Jackel M, Kerenyi T, Kadar A. Tenascin expression and angiogenesis in breast cancers. Pathol Res Pract 1999;195:821–28
Acknowledgements
We would like to thank Karen Nelson for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beecken, WD.C., Engl, T., Ringel, E.M. et al. An Endogenous Inhibitor of Angiogenesis derived from a Transitional Cell Carcinoma: Clipped β2-Glycoprotein-I. Ann Surg Oncol 13, 1241–1251 (2006). https://doi.org/10.1245/s10434-006-9009-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9009-9