-
PDF
- Split View
-
Views
-
Cite
Cite
Odile Paulmyer-Lacroix, Sandrine Boullu, Charles Oliver, Marie-Christine Alessi, Michel Grino, Expression of the mRNA Coding for 11β-Hydroxysteroid Dehydrogenase Type 1 in Adipose Tissue from Obese Patients: An in Situ Hybridization Study, The Journal of Clinical Endocrinology & Metabolism, Volume 87, Issue 6, 1 June 2002, Pages 2701–2705, https://doi.org/10.1210/jcem.87.6.8614
Close - Share Icon Share
Glucocorticoids play an important role in determining adipose tissue metabolism and distribution. Patients with Cushing’s syndrome or receiving corticosteroid therapy develop a reversible visceral obesity. In obese patients, although circulating concentrations of cortisol are not consistently elevated, local conversion of inactive cortisone to active cortisol in adipose tissue, catalyzed by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD-1), could amplify glucocorticoid signaling. We have studied, using semiquantitative in situ hybridization, 11β-HSD-1 mRNA expression in the adipocyte and stromal compartments of sc abdominal adipose tissue obtained from 12 lean patients and sc abdominal and visceral adipose tissue obtained from 18 obese patients. 11β-HSD-1 mRNA was expressed in adipocytes, stroma, and walls of vessels. Localization of 11β-HSD-1 mRNA did not differ between lean sc and obese sc or visceral adipose tissue. 11β-HSD-1 mRNA levels were significantly (P = 0.0106) increased in the adipocyte compartment of sc adipose tissue obtained from obese patients as compared with nonobese ones, whereas no significant change (P = 0.446) was found in the stromal compartment. In obese patients, 11β-HSD-1 mRNA expression was increased (P = 0.0157) in the stromal compartment of visceral compared with sc tissue, whereas no significant change (P = 0.8767) was found in the adipocyte compartment.
In summary, our data show that 11β-HSD-1 mRNA is increased in adipose tissue from obese patients, in the abdominal sc fat in adipocytes and in the visceral fat in both adipocytes and stroma. This observation suggests that an overexpression of 11β-HSD-1 may explain part of the glucocorticoid-induced metabolic disorders linked to obesity and may promote visceral fat deposition.
OBESITY IS ASSOCIATED with health risks such as hypertension, coronary artery disease, hyperlipidemia, and diabetes mellitus (1). These complications are more frequent when adipose tissue is deposited in the truncal region, in particular at a visceral level, as compared with generalized obesity (2). The factors responsible for an increased visceral localization of adipose tissue are not completely understood. However, cortisol was implicated as a pathophysiological mediator. Indeed, patients with Cushing’s syndrome develop a reversible visceral obesity (3). In patients suffering from visceral obesity, circulating concentrations of cortisol are not consistently elevated (4). An increase in glucocorticoid receptors in visceral fat could locally amplify glucocorticoid signaling (5). Alterations in peripheral cortisol metabolism together with a compensatory increase in cortisol secretion could also play a role (6). The changes in peripheral cortisol metabolism could associate an increase in 5α-reductase conversion of cortisol to 5α-tetrahydrocortisol and a reduction of type 1 11β-hydroxysteroid dehydrogenase (11β-HSD-1) reactivation of cortisone to cortisol by the liver (7, 8). In addition, increased generation of cortisol may take place in adipose tissue because 11β-HSD-1 immunoreactivity was observed in omental adipose tissue, in both stromal and adipocytes cells (9). Studies in obese Zucker rat have demonstrated an increased 11β-HSD-1 activity in omental adipose tissue (10). Transgenic mice overexpressing 11β-HSD-1 selectively in adipose tissue have increased adipose levels of corticosterone, develop visceral obesity, and are glucose intolerant (11). However, the possibility of tissue-specific dysregulation of 11β-HSD-1 is difficult to explore in human studies. Katz et al. (12) have suggested, using arteriovenous sampling, that 11β-HSD-1 activity in sc abdominal adipose tissue is increased in obesity. It has been demonstrated in vitro that:
11β-HSD-1 activity was higher in visceral (omental) compared with sc adipose stromal cells obtained from nonobese patients, both under basal or stimulated conditions (13, 14); and basal 11β-HSD-1 activity was higher in whole sc abdominal adipose tissue explants obtained from obese patients as compared with normal controls (15).
However, it is not clear whether: 11β-HSD-1 expression is increased in visceral adipose tissue in obese patients; variations in 11β-HSD-1 expression in either sc or visceral adipose tissue are restricted to the stromal compartment, and therefore can affect the differentiation of preadipocytes to adipocytes, or include the adipocyte compartment, and as a consequence can modulate the adipocyte metabolism, in particular its insulin sensitivity.
Using semiquantitative in situ hybridization, we studied the expression of the mRNA coding for 11β-HSD-1 in the adipocyte and stromal compartments of sc abdominal adipose tissue obtained from lean patients and of sc abdominal and visceral adipose tissue obtained from obese patients.
Materials and Methods
Tissue samples
The study was conducted in accordance with the guidelines proposed in The Declaration of Helsinki and was approved by the local hospital and university ethics committee. All subjects neither suffered from any ongoing disease nor received any endocrine therapy. Abdominal adipose sc tissue was obtained from 12 female patients during abdominal lipectomy (body mass index: mean ± sd: 23 ± 3.7 kg/m2 and age: 33 ± 10 yr). Visceral and sc abdominal adipose tissues were obtained during gastroplasty from 18 patients (15 females, 3 males; body mass index: 41 ± 6.3 kg/m2, age: 41 ± 12 yr). Tissues were immediately frozen on dry ice and stored at −70 C.
In situ hybridization
Twenty-micrometer sections were cut in a cryostat, mounted on gelatin-coated slides, and processed and hybridized as previously described (16). The probe was a 356-bp cDNA fragment corresponding to base 27 of exon 3-base 104 of exon 5 of the human 11β-HSD-1 gene (17), subcloned in pPCR script, linearized with BamHI (antisense probe) or with BstXI (sense probe) and labeled with 35S-UTP (Perkin-Elmer Corp., France). After hybridization and washing, slides were dipped in nuclear emulsion (Ilford K5) diluted 1:2 in water and exposed for one month. After development, sections were counterstained with neutral red.
Brightfield images were captured with a color charge-coupled device camera (Coolsnap, Princeton Instruments, France) attached to a Leica Corp. (Rueil-Malmaison, France) microscope and digitized. Because there was no significant variation in grain density between all the areas studied in both sc and visceral adipose tissue, semiquantitative analysis was performed by measuring the surface of the hybridized areas using the Image software (18). Eight randomly chosen fields (0.7 mm2) per section were analyzed. The resulting average values were analyzed using the Mann-Whitney U test (to compare 11β-HSD-1 mRNA expression in sc tissue between lean and obese patients) or the paired Wilcoxon test (to compare 11β-HSD-1 mRNA expression between sc and visceral tissue in obese patients) using the Statview analysis program. All data are presented as the mean ± se.
Results
Localization of 11β-HSD-1 mRNA
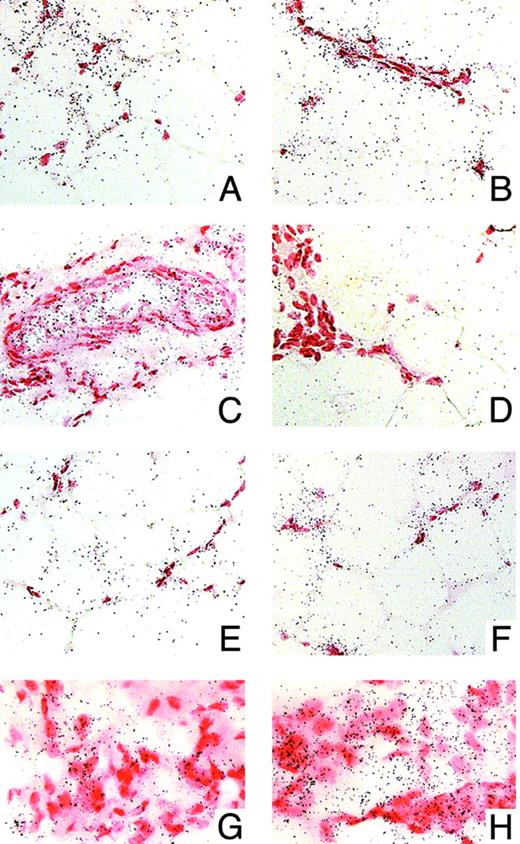
Figure 1, A–D, shows a brightfield view of an in situ hybridization of 11β-HSD-1 mRNA in sc abdominal adipose tissue obtained from a lean patient. After hybridization with the 11β-HSD-1 antisense probe, an intense labeling was found in adipocytes and in the stromal compartment (stromal cell clusters, isolated stromal cells closed to adipocytes, and walls of vessels; Fig. 1, A–C, respectively). Hybridization with the sense probe did not give any detectable signal, demonstrating the specificity of the hybridization (Fig. 1D).
Brightfield view of in situ hybridization for 11β-HSD-1 mRNA in adipose tissue from control or obese patients. Sections were counterstained with neutral red. Signal appears as silver grains. A–D, Expression of 11β-HSD-1 mRNA in sc adipose tissue from a lean patient. A–C, Hybridization with a antisense probe: grain clusters are present over adipocytes (A), stroma (B), and walls of vessels (C). D shows the result of a hybridization with a sense probe: note the lack of grain clusters, demonstrating the specificity of the probe. E and F, expression of 11β-HSD-1 mRNA in the adipocytes compartment of sc adipose tissue obtained from a lean (E) or an obese (F) patient. G and H, Expression of 11β-HSD-1 mRNA in the stromal compartment of sc (G) or visceral (H) adipose tissue obtained from an obese patient. Bar, 50 μm in A–F and 25 μm in G and H.
Regulation of 11β-HSD-1 mRNA expression
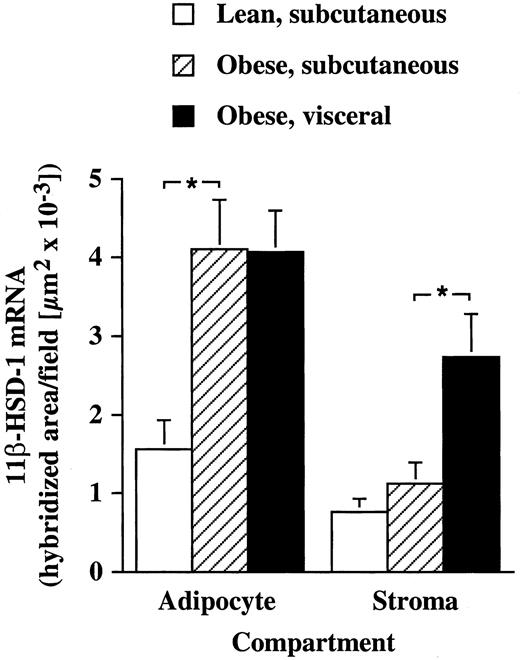
The localization of 11β-HSD-1 mRNA did not differ between nonobese sc and obese sc or visceral adipose tissue (not shown). Figure 2 shows the results of the semiquantitative analysis of 11β-HSD-1 mRNA expression in the adipocyte or stromal compartments of sc or visceral adipose tissue obtained from lean or obese patients. 11β-HSD-1 mRNA levels were significantly (P = 0.0106) increased in the adipocyte compartment of sc adipose tissue obtained from obese patients as compared with non obese ones (Fig. 1, E and F), whereas no significant change (P = 0.446) was found in the stromal compartment. In obese patients, 11β-HSD-1 mRNA expression was increased (P = 0.0157) in the stromal compartment of visceral compared with sc tissue (Fig. 1, G and H), whereas no significant change (P = 0.8767) was found in the adipocyte compartment.
Semiquantitative analysis of in situ hybridization for 11β-HSD-1 mRNA in adipocytes or stromal compartments of sc or visceral adipose tissue obtained from lean (n = 12) or obese (n = 18) patients. *, P < 0.05.
Discussion
Our data demonstrate that 11β-HSD-1 mRNA levels are elevated in adipose tissue obtained from obese patients, suggesting the existence of an increased local conversion of cortisone to cortisol. Indeed, it has been demonstrated that there is a parallelism between 11β-HSD-1 activity and mRNA levels (19). This observation extends previous findings, obtained in vivo or in vitro (13–15). In addition, our data evidence that the regulation of 11β-HSD-1 gene expression differs between the localization (sc or visceral) and the compartment (stroma or adipocytes) studied. This may explain apparent discrepancies in previously published papers. Indeed, Bujalska et al. (13, 14), using cultured adipose stromal cells, suggested that local conversion of cortisone to cortisol occurs mainly in visceral rather than in sc adipose tissue, whereas Katz et al. (12) and Rask et al. (15) demonstrated that sc abdominal adipose tissue shows a robust 11β-HSD-1 activity. Our finding that sc adipocytes, a compartment that was not taken in account in Bujalska’s studies (13, 14), express significant amount of 11β-HSD-1 mRNA could explain these differences.
Stromal 11β-HSD-1 mRNA was higher in visceral compared with sc adipose tissue obtained from obese patients. This result extends the findings reported by Bujalska et al. (13) in nonobese subjects and suggests that obese patients have also an increased local conversion of cortisone to cortisol in visceral fat. Such a phenomenon may promote an accumulation of visceral adipose tissue. Indeed, an increase in visceral fat has been well documented in patients with Cushing’s syndrome or receiving corticosteroid therapy (3). In vitro, glucocorticoids are required for the differentiation of stromal cells to mature adipocytes (20, 21). Glucocorticoids modulate lipoprotein lipase and glucose-3-phosphate dehydrogenase, inducing an increase in lipid accumulation in primary cultures of stromal cells (22, 23) and in the 3T3-L1 preadipocyte cell line (24). The increase in 11β-HSD-1 mRNA in visceral stromal cells of obese patients can exacerbate this phenomenon.
Our results demonstrate that 11β-HSD-1 mRNA is increased in the adipocyte compartment of both sc and visceral adipose tissue from obese patients and suggest that the subsequent increased local conversion of cortisone to cortisol could influence adipocytes metabolism. It is known that, in vitro, cortisol increases lipoprotein lipase (25) and hormone-sensitive lipase (26) activity in adipose tissue. As a consequence, an excessive release of free fatty acids and glycerol from the adipocytes into the circulation can lead to insulin resistance. The increased expression of 11β-HSD-1 in visceral adipose tissue is in line with the general consensus that visceral fat plays an important role in the metabolic disturbances induced by obesity. It has been shown, in moderately obese rats, that surgical removal of visceral fat improves insulin sensitivity (27). However, we found that 11β-HSD-1 mRNA levels were also elevated in the sc abdominal adipocytes compartment from obese patients. This observation is consistent with the findings of Katz et al. (12) and Rask et al. (15) and suggests that the increased 11β-HSD-1 expression in sc abdominal adipose tissue may participate in the metabolic disturbances induced by obesity. Interestingly, some groups have reported that, in vivo, sc abdominal fat was at least as strong a correlate of insulin sensitivity as visceral fat (28, 29).
The mechanisms responsible for the tissue-specific regulation of 11β-HSD-1 gene expression are not completely understood. Factors influencing 11β-HSD-1 expression include glucocorticoids, thyroid hormones, sex steroids, GH, IGF-1, insulin, cytokines, and synthetic factors such as PPARγ ligands (30). Glucocorticoids- or GH-induced modulation of 11β-HSD-1 could be involved the accumulation of adipose tissue and the dysregulation of its metabolism seen in obesity, whereas part of the therapeutic effects of PPARγ ligands may take place through their action on 11β-HSD-1. In vitro in primary cultures of human omental or sc adipose stromal cells, glucocorticoids are able to elicit a fast forward feedback in a dose-dependent fashion (14). A similar phenomenon could occur in adipocytes because they express glucocorticoid receptors (31). Obese patients have decreased GH secretion (32). Interestingly, it is known that administration of daily GH to hypopituitary patients results in lower ratios of cortisol/cortisone metabolites consistent with inhibition of 11β-HSD-1 (33). Several recent observations have suggested a role for PPARγ in controlling 11β-HSD-1 expression in adipose tissue. Rosiglitazone inhibited both 11β-HSD-1 activity and mRNA expression in 3T3-L1 cells and in visceral adipose tissue from db/db diabetic mice (19), and, in humans, treatment with troglitazone decreases visceral fat mass (34, 35). These phenomenon may mediate some of the antidiabetic effects of PPARγ agonists.
In summary, our data show that 11β-HSD-1 mRNA is increased in adipose tissue from obese patients, in the sc fat in adipocytes, and in the visceral fat in both adipocytes and stroma. This observation suggests that an overexpression of 11β-HSD-1 may explain part of the glucocorticoid-induced metabolic disorders linked to obesity and may promote visceral fat deposition. Specific inhibitors of 11β-HSD-1 may be useful for the treatment of patients with obesity (15).