-
PDF
- Split View
-
Views
-
Cite
Cite
Md Azadul Kabir Sarker, Sho Aki, Kazuaki Yoshioka, Kouji Kuno, Yasuo Okamoto, Kazuhiro Ishimaru, Noriko Takuwa, Yoh Takuwa, Class II PI3Ks α and β Are Required for Rho-Dependent Uterine Smooth Muscle Contraction and Parturition in Mice, Endocrinology, Volume 160, Issue 1, January 2019, Pages 235–248, https://doi.org/10.1210/en.2018-00756
Close - Share Icon Share
Abstract
Class II phosphoinositide 3-kinases (PI3Ks), PI3K-C2α and PI3K-C2β, are highly homologous and distinct from class I and class III PI3Ks in catalytic products and domain structures. In contrast to class I and class III PI3Ks, physiological roles of PI3K-C2α and PI3K-C2β are not fully understood. Because we previously demonstrated that PI3K-C2α is involved in vascular smooth muscle contraction, we studied the phenotypes of smooth muscle–specific knockout (KO) mice of PI3K-C2α and PI3K-C2β. The pup numbers born from single PI3K-C2α–KO and single PI3K-C2β–KO mothers were similar to those of control mothers, but those from double KO (DKO) mothers were smaller compared with control mice. However, the number of intrauterine fetuses in pregnant DKO mothers was similar to that in control mice. Both spontaneous and oxytocin-induced contraction of isolated uterine smooth muscle (USM) strips was diminished in DKO mice but not in either of the single KO mice, compared with control mice. Furthermore, contraction of USM of DKO mice was less sensitive to a Rho kinase inhibitor. Mechanistically, the extent of oxytocin-induced myosin light chain phosphorylation was greatly reduced in USM from DKO mice compared with control mice. The oxytocin-induced rise in the intracellular Ca2+ concentration in USM was similar in DKO and control mice. However, Rho activation in the intracellular compartment was substantially attenuated in DKO mice compared with control mice, as evaluated by fluorescence resonance energy transfer imaging technique. These data indicate that both PI3K-C2α and PI3K-C2β are required for normal USM contraction and parturition mainly through their involvement in Rho activation.
Reproduction is highly coordinated by various hormones and local mediators with the integration by the neuroendocrine system (1, 2). The uterus, one of the most important female reproductive organs, accommodates and nurtures the growing fetus (3). The uterus comprises three layers: the endometrial, myometrial, and outer serosal layers. During pregnancy, the myometrium remains quiescent and undergoes enormous hyperplasia and hypertrophy to provide a proper environment for fetal growth and to prepare for generation of a uterine contractile force sufficient for fetal delivery. At the last stage of pregnancy, stretching of the uterine wall by the grown-up fetus and other changes in the local uterine environment cause activation of the myometrium, bringing about myometrial alterations, including the extreme upregulation of the oxytocin receptor and voltage-dependent Ca2+ channel (4, 5).
Phosphoinositide 3-kinases (PI3Ks) are lipid kinases that catalyze the phosphorylation at the 3′-hydroxyl group of the inositol ring in phosphoinositides (6). PI3Ks comprise three classes of PI3Ks. Class I PI3Ks, p110α, p110β, p110γ, and p110δ, are activated downstream of various receptor tyrosine kinases and G protein–coupled receptors to mainly generate phosphatidylinositol 3,4,5-trisphosphate and to mediate cell proliferation, survival, and migration. Class III PI3K, Vps34, mainly regulates autophagy by generating phosphatidylinositol 3-phosphate. Class II PI3Ks comprise PI3K-C2α (C2α), PI3K-C2β (C2β), and PI3K-C2γ, which generate phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2) and probably phosphatidylinositol 3-phosphate (7–9). C2α and C2β are ubiquitously expressed widely in various organs and tissues whereas PI3K-C2γ expression is restricted mainly to liver, breast, testis, and prostate (10). In contrast to class I and class III PI3Ks, the physiological function of class II PI3Ks was not well understood.
We previously demonstrated using small interfering RNA–mediated specific knockdown technique that C2α was required for noradrenaline- and ionomycin-induced contraction of vascular smooth muscle cells (11–13). Subsequently, we generated C2α-knockout (KO) mice to study a role of C2α at an organismal level and found that homozygous C2α-KO mice were embryonic lethal owing to severe vascular endothelial defects (14). C2α-deficient vascular endothelial cells showed impairment of Rho activation in the endosomes in response to the angiogenic factor vascular endothelial growth factor (VEGF) due to defects of VEGF receptor internalization, which led to impaired proliferation, migration, and cell–cell adhesion of endothelial cells (14). Furthermore, we observed in endothelial cells that endosomal activation of Rac and Smad2/3 was also impaired upon stimulation by sphingosine-1-phosphate (S1P) and TGFβ1, respectively, owing to defects in the internalization of the S1P receptor and TGFβ1 receptor (9, 15). These findings suggested that C2α is required for receptor endocytosis and subsequent receptor signaling in the endosomes for at least certain receptor ligands in endothelial cells. C2β is highly homologous in its amino acid sequence to C2α and exhibits similar activities, including cell migration and growth, compared with C2α. However, it is not well known how important C2β is in endocytosis and the intracellular signaling (16).
In the current study, we sought to reveal physiological roles of C2α and C2β in smooth muscle organs by generating smooth muscle–specific KO mice of C2α and C2β. During this study, we found that pregnant smooth muscle–specific double KO (smDKO) female mice, but not single KO mice of either C2α or C2β, delivered smaller numbers of pups compared with control mice, although the numbers of fetuses in the uteri at the term pregnancy did not differ between smDKO and control mice. Isolated uterine smooth muscle (USM) from smDKO mice showed attenuated contraction with reduced intracellular Rho activation when compared with control mice. These results indicate the novel physiological role of C2α and C2β in USM contraction and pup delivery.
Materials and Methods
Materials
Advanced DMEM (Gibco, catalog no. 12491-015) and FluoroBrite DMEM (Gibco, catalog no. A1896701) were purchased from Gibco/Life Technologies (Grand Island, NY). Fluo-8 AM (catalog no. 21081) was purchased from AAT Bioquest (Sunnyvale, CA). Liberase TM (catalog no. 5401127001) and protease inhibitor cocktail cOmplete mini (catalog no. 11836153001) were purchased from Roche (Mannheim, Germany). Y-27632 (catalog no. 253-00513) and normal goat serum (catalog no. 143-06561) were purchased from Wako Pure Chemicals Industries (Osaka, Japan). Oxytocin (catalog no. O6379), nitrendipine (catalog no. N144), and other chemicals were bought from Sigma-Aldrich (St. Louis, MO) unless specified otherwise. The following antibodies were used in this study: rabbit polyclonal C2α (catalog no. AP1155B; Abgent, San Diego, CA), rabbit polyclonal C2β (catalog no. sc-134766; Santa Cruz Biotechnology, Dallas, TX), rabbit polyclonal anti–smooth muscle myosin heavy chain 11 (catalog no. ab53219; Abcam, Cambridge, United Kingdom), mouse monoclonal anti-caldesmon (catalog no. C-6542; Sigma-Aldrich), monoclonal anti–myosin light chain (MLC) kinase (MLCK) (catalog no. M7905; Sigma-Aldrich), rabbit polyclonal PP1δ antibody (17), mouse monoclonal anti-calponin (catalog no. C2687; Sigma-Aldrich), rabbit polyclonal phosphorylated myosin phosphatase target subunit-1 (MYPT1) (Thr853) (catalog no. 4563; Cell Signaling Technologies, Danvers, MA), mouse monoclonal anti-MYPT1 (catalog no. 612165; BD Biosciences, San Jose, CA), rabbit monoclonal C2α (D3Q5B) from Cell Signaling Technologies (ref. no. 12402), mouse monoclonal anti-C2β antibody from BD Biosciences (San Jose, CA; material no. 611342), rabbit polyclonal phosphorylated 20-kDa MLC (MLC20) (Ser19) (catalog no. 3671; Cell Signaling Technologies), mouse monoclonal anti-MLC (product no. M4401; Sigma-Aldrich), mouse monoclonal anti-actin, α-smooth muscle (catalog no. A5228; Sigma-Aldrich), rabbit polyclonal diphosphorylated MLC20 (Thr18/Ser19) [from Dr. M. Seto in Asahi Chemical Industry (Fuji, Japan)], mouse monoclonal anti-calcium channel L-type DHPR α subunit (Cavα2) (catalog no. ab2864; Abcam), and mouse monoclonal anti-Slo1 (BKα1) (catalog no. MABN70; EMD Millipore, Billerica, MA).
Mice
All animal experiments were conducted according to the “fundamental guidelines for proper conduct of animal experiment and related activities in academic research institutions” under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology of Japan and were approved by the Committee on Animal Experimentation of Kanazawa University. C2α-floxed (C2αfl/fl) mice were described previously (14). C2β mice that carry three loxP sequences, one loxP before exon 3 and two loxP sequences flanking the neomycin cassette in the recombined Pik3c2b gene (C2β3lox/3lox mice) (18), were obtained from The Jackson Laboratory (Bar Harbor, ME; B6.129-Pik3c2btm1Pkha/J, stock no. 005702) and are described elsewhere (19). To generate mice with a Pi3kc2b conditional allele that carries two loxP sites (C2βfl/fl mice), C2β3lox/3lox mice were crossed with insulin–Cre recombinase (Cre) transgenic mice to delete the neomycin cassette (14, 18). To generate Pi3kc2b-null (C2β−/−) mice, the three loxP–targeted allele was removed by intercrossing the CAG-Cre transgenic mice line (18, 20). To generate smooth muscle–specific Pik3c2b conditional KO (C2βfl/fl;SM22α-Cre) mice, C2βfl/fl mice were crossed with SM22α-Cre transgenic mice (18, 21). In some experiments, C2β3lox/3lox mice were crossed with SM22α-Cre transgenic mice to generate smooth muscle–specific C2β−/− mice (18). In the present study, we employed three genetically different mice with C2α and C2β DKO in smooth muscle, that is, C2αfl/fl;C2βfl/fl;SM22α-Cre, C2αfl/fl;C2β3lox/3lox;SM22α-Cre, and C2αfl/fl;C2β−/−;SM22α-Cre mice (18), to study the roles of C2α and C2β. Rosa26-CAG-loxP-stop-loxP-tdTomato (R26-tdTomato) reporter mice were from The Jackson Laboratory (B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, stock no. 007908). SM22α-Cre;R26-tdTomato mice were generated to evaluate Cre-mediated recombination efficiency. Mice were euthanized using IP injection of pentobarbital (Kyoritsu, Tokyo, Japan) overdose according to the acceptable euthanasia guidelines. Mice were genotyped by PCR analysis of genomic DNA prepared from tail biopsies.
Counting pups and intrauterine fetuses
We performed timed mating of mice. Mouse mating was performed in 1:1 ratio by placing one female mouse into a cage containing one male mouse at late afternoon at gestational day (GD) 0. The following morning, all female mice, whether they were containing a vaginal plug or not, were separated from male mice and followed until GD18.5 or delivery. We counted delivered pups on the morning of the expected day of delivery. In some pregnant female mice, we carefully monitored the numbers of pups delivered from each mouse every 3 hours on the expected delivery day. For counting intrauterine fetuses in the fertility test, mice were euthanized at GD18.5 with overdose of pentobarbital and intrauterine fetuses were counted. In the fertility test, 12 control and 12 DKO mice were used and the data were from five litters in both control and smDKO female mice.
Western blotting
For preparation of nonstimulated myometrium tissue lysate, the myometrium layer was quickly dissected from the isolated uterus by removing the endometrium and placenta after euthanizing mice. The myometrial tissues were snap frozen by soaking them in liquid nitrogen. Frozen tissues were quickly homogenized with glass homogenizer in ice-cold modified RIPA buffer [10 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na2HPO4, 2 mM Na3VO4, 0.1% SDS, 0.05% sodium deoxycholate, 1% Triton X-100, 10% glycerol, and one tablet of cOmplete mini per 10 mL of buffer) or kept at −80°C for storage. After debris was removed, the samples were solubilized in 2× Laemmli SDS sample buffer, boiled for 5 minutes, and then separated on 8% or 14% SDS-PAGE, followed by electrotransfer to polyvinylidene difluoride membranes (Immobilon-P; Millipore-Merck, Darmstadt, Germany). After blocking with 5% BSA for 1 hour, the membranes were incubated overnight with different antibodies at 4°C, followed by incubation with alkaline phosphatase–conjugated secondary antibodies for 1 hour at room temperature. Protein bands were visualized by color reaction using the nitro blue tetrazolium/5-bromo-4-chloro-3′-indolyl phosphate p-toluidine system. The band densities of different proteins were determined using Image Studio lite software (LI-COR Biotechnology).
Immunofluorescent staining
Cryosections of uterus were stained for immunofluorescence observations using a standard protocol as described previously (14). Briefly, after overnight fixation in 4% paraformaldehyde in Ca2+- and Mg2+-free PBS, the tissues were washed several times with PBS, cryoprotected in 20% sucrose for 12 to 16 hours, and snap frozen with Tissue-Tek OCT compound (Sakura, Tokyo, Japan). Tissue sections (7 µm) were prepared by using Sakura Tissue-Tek Cryo3 and the sections were blocked with 5% normal goat serum in 0.3% Triton X-100/PBS for 1 hour at room temperature. Primary antibodies were applied at the indicated dilutions [anti-C2α (1:100), anti- C2β (1:200), anti–myosin heavy chain 11 (Mhc11) (1:250), Cavα2 (1:100), and anti-Slo1 (BKα1) (1:150)] and incubated for 12 to 16 hours at 4°C. After several washes with PBS, Alexa Fluor 488–conjugated anti-rabbit IgG (1:1000) was applied to the sections and incubated at room temperature for 2 hours. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 30 minutes and mounted with Fluoromount (Diagnostic BioSystems, Pleasanton, CA) with coverslips. Confocal microscopic observations were carried out on an inverted Nikon Eclipse Ti2 confocal microscope (Nikon Instruments), attached to an Andor Dragonfly spinning-disk unit, Andor EMCCD camera (iXon DU888) (Andor Technologies), and a laser unit (Coherent). An oil-immersion objective (PlanApo ×60, numerical aperture 1.4; Nikon) was used for all experiments. Excitation for DAPI, Alexa Fluor 488, and Alexa Fluor 568 chromophores was provided by 405-, 488-, and 561-nm lasers, respectively. Super-resolution imaging of fixed cells was performed using an Andor Dragonfly confocal microscope in super-resolution radial fluctuation (SRRF)–Stream mode.
Measurements of tension and phosphorylation of MLC20 and MYPT1
Tension measurements were performed as described previously (22). In brief, isolated uteri were placed immediately in ice-cold Krebs–Henseleit buffer composed of 119 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.5 mM MgSO4, 1.5 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose. The buffer containing uterine tissues was continuously aerated with 95% O2 and 5% CO2. Uteri were cleaned of fetus, placenta, and other adherent tissues and made into strips of ∼2.5 × 15 mm. After placing strips in contraction chambers, generated tension was determined isometrically with a transducer (UM-203; Kishimoto Medical Instruments, Kyoto, Japan) and strips were continuously aerated with 95% O2 and 5% CO2. Before test stimulation, the rings were precontracted with 60 mM KCl–containing buffer, and poorly responsive strips were not used for analyses. Spontaneous tension and oxytocin- and KCl-induced tension were corrected by the tension generated by the application of a hypotonic buffer at the end of tension measurements as described previously (23, 24). The optimal concentrations of oxytocin, Y-27632, and nitrendipine were determined by preliminary experiments.
Uterine strips contracted isometrically were fixed in acetone dry ice slurry containing 20 mM dithiothreitol and 10% trichloroacetic acid and washed in acetone containing 10 mM dithiothreitol at room temperature, as described previously (12). Fixed tissues were homogenized in a homogenization buffer comprised of 20 mM Tris/HCl (pH 7.5), 100 mM NaF, 1 mM Na3VO4, 0.1% SDS, 2 mM EGTA, 0.5% Nonidet P-40, 20 μg/mL each of leupeptin and aprotinin, and 1 mM phenylmethylsulfonyl fluoride. The homogenates were mixed with 4× Laemmli SDS sample buffer and boiled for 5 minutes. The samples (40 μg of protein) were separated on 8% and 14% SDS-PAGE, followed by western blot analysis using phosphorylated MLC20- and MYPT1-specific antibodies and antibodies that recognize both phosphorylated and nonphosphorylated proteins. The amounts of the phosphorylated proteins quantitated by densitometry were normalized for total amounts of MLC20 and MYPT1 in each sample, and the quantified data of normalized amounts of the phosphorylated proteins were expressed as multiples over a value in unstimulated tissues, which is expressed as 1.0.
Isolation and culture of myometrial cells
Myometrial cells were isolated and cultured as described previously (25). Briefly, the uterus quickly removed from euthanized mice was washed with ice-cold Hanks' balanced salt solution (HBSS) and cut through the longitudinal axis. The placenta, fetus, and endometrium were removed from the uterus and the myometrium layer was finely chopped with scissors. The myometrial tissues were incubated in 1 U/ml Liberase TM in HBSS solution for 1 hour at 37°C. Cell suspensions were prepared by gently pipetting 15 to 20 times with penicillin G– and strepromycin-supplemented, 5% FBS-containing advanced DMEM and filtered through a 100-µm cell strainer (catalog no. 352360; Falcon, Corning, NY). Cell suspensions were centrifuged at 450 × g for 5 minutes at 20°C, and the resultant cell pellets were resuspended in penicillin G– and strepromycin-supplemented, 5% FBS-containing advanced DMEM and plated onto type I collagen (Nitta Gelatin)–coated dishes.
Measurements of the intracellular free Ca2+ concentration
For measurements of the intracellular free Ca2+ concentration [Ca2+]i, freshly isolated myometrial smooth muscle cells were seeded on type I collagen–coated glass-bottomed culture dishes (catalog no. P35G-1.0-14-C; MatTek Corporation) and kept for 18 to 20 hours at 37°C in atmosphere containing 5% CO2. Cells were washed with prewarmed HBSS and loaded by 5 µM Fluo-8 am in HBSS for 30 minutes. After washing twice with HBSS, cells were incubated in phenol red–free FluoroBrite DMEM (Gibco) on a heated stage chamber at 37°C and 5% CO2 (Tokai Hit). The intracellular Ca2+ imaging was performed using a customized inverted microscope (IX70; Olympus)–based spinning disk (CSU-10; Yokogawa) confocal system, equipped with an EMCCD cooled camera (iXon, Andor, United Kingdom) and a light engine (Lumencor). Fluorescent images were captured every 500 ms with excitation at 488 nm light and fluorescence detection at 510 nm. Pixel density was calculated from whole-cell averages using the iXon iQ software (Andor). The ratio of oxytocin-stimulated peak fluorescence intensity/basal intensity was expressed.
Determination of Rho activation by fluorescence resonance energy transfer imaging technique
For fluorescence resonance energy transfer (FRET) imaging analysis, after myometrial smooth muscle cells were isolated, they were transfected with the pRaichu-RhoA probe (26) using an Amaxa Nucleofector system (Lonza) and plated onto type I collagen–coated, glass-bottomed culture dishes. Twenty-four hours later, the transfected cells were imaged using the same basic confocal microscope system as described for the [Ca2+]i measurements. For the measurements of Rho-FRET signals, the confocal system was configured with a cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) filter set (Di01-T445/515/561-13×15×0.5; Semrock). The employed chimeric FRET probe protein consists of N-terminal YFP, the Rho-binding domain of PKN, RhoA, and C-terminal CFP. When RhoA in the chimeric FRET probe protein is bound to GDP, fluorescence of 475 nm emanates from CFP with excitation at 433 nm. When RhoA is bound to GTP, intramolecular binding of GTP-loaded RhoA to the RBD brings YFP into close proximity to CFP, which causes FRET and fluorescence of 527 nm from YFP. The chimeric FRET probe–transfected cells were stimulated with oxytocin (100 nM), which was added after 2-minute observations of baseline signals. Pseudo-grayscale ratio images were generated from images from CFP and FRET channels using Andor iQ software. RhoA FRET signal intensity within four subcellular regions per cell at 3 minutes after the addition of oxytocin was quantified. The ratio of oxytocin-stimulated fluorescence intensity/basal intensity was expressed.
Statistical analysis
Statistical analysis and graphical presentation were performed with GraphPad Prism 7 software (GraphPad Software). Data are presented as means ± SEM. Analysis between two groups was done with a two-tailed unpaired Student t test. For comparisons between multiple groups, one- or two-way ANOVAs followed by a Bonferroni post hoc test were used unless stated otherwise. A P value <0.05 was considered to be statistically significant.
Results
Genetic deletion of C2α and C2β in female mice impedes fetal delivery
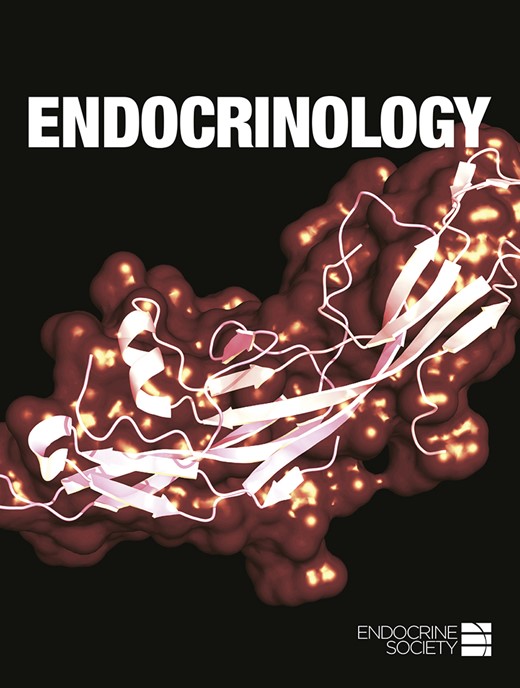
To study roles of C2α and C2β in the smooth muscle organs, we generated smooth muscle–specific KO mice of C2α and C2β by mating C2α- and C2β-floxed mice with SM22α-Cre transgenic mice. In this study, we used two different types of C2β-floxed mice, that is, C2βfl/fl and C2β3lox/3lox mice (18), to delete the C2β gene specifically in smooth muscle tissues. We confirmed that SM22α promoter–driven Cre expression effectively deleted the floxed gene in the R26-tdTomato reporter construct in smooth muscle tissues, including the uterus and bladder (Fig. 1A). SM22α promoter–driven Cre expression substantially decreased the protein expression of both C2α and C2β in the myometrial layer of the uterus (Fig. 1B) of smDKO mice with the genotype of C2αfl/fl;C2βfl/fl;SM22α-Cre. While we were conducting the mating, we found that female smDKO mice in the mating [C2αfl/fl;C2βfl/fl;SM22α-Cre (female) × C2αfl/fl;C2βfl/fl (male)] delivered reduced numbers of pups compared with two other matings [C2αfl/fl;C2βfl/fl (female) × C2αfl/fl;C2βfl/fl;SM22α-Cre (male), and C2αfl/fl;C2βfl/fl (female) × C2αfl/fl;C2βfl/fl (male)] (Table 1). We also observed a reduction in the number of pups from female C2αfl/fl;C2β−/−;SM22α-Cre mice, in which C2β is globally deleted, compared with female C2αfl/fl;C2β−/− mice (Table 2). C2β3lox/3lox mice expressed ∼80% of the C2β protein expression level in smooth muscle tissues of wild-type mice. The pup number from female C2αfl/fl;C2β3lox/3lox;SM22α-Cre mice was also reduced compared with female wild-type and C2αfl/fl;C2β3lox/3lox mice (Table 3). In contrast, single smooth muscle–specific KO of C2α (smC2αKO) or C2β (smC2βKO) in female mice did not reduce pup numbers (Table 3). We followed the time course of the delivery in pregnant mice: both control (C2αfl/fl;C2βfl/fl) and smDKO mice started delivery at night or early morning. The control mice finished the delivery process by 1500 hours whereas smDKO mice delivered the small number of pups by 1500 hours and thereafter smDKO mice did not deliver pups or delivered only a few pups by 2400 hours (Fig. 1C). Regardless of the decreased pups delivered from smDKO mice, the number of fetuses at GD18.5 in the uterus of smDKO mice was not different from that of control C2αfl/fl;C2βfl/fl mice (Fig. 1D). The gross fetal appearance, including the attachment and orientation of fetuses to the placenta in smDKO pregnant mice, was also similar to that in control mice. These observations suggested that the fetal delivery process was impaired in female smDKO mice.
Impaired pup delivery in smooth muscle–specific C2α and C2β DKO mice. (A) Immunofluorescent staining of Mhc11 in the smooth muscle layer of uterus and bladder in SM22α-Cre;R26-tdTomato reporter mice. Mhc11-positive cells express tdTomato protein. The schematic for generation of mice with smooth muscle–specific expression of tdTomato fluorescent protein is shown on the top. (B) Immunofluorescent staining of C2α and C2β in the uterine myometrium of control and smDKO pregnant mice at GD18.5. (C) Time course of pup delivery from control and smDKO female mice. (D) Uteri of pregnant mice and the numbers of pups within the uteri at GD18.5. The magnified views of the boxed portion of the uteri in the leftmost images are shown in (i) and (ii). In (C) and (D), the numbers in the columns denote the numbers of analyzed pregnant mice. The data in (C) and (D) are expressed as means ± SEM. *P < 0.05. EM, endometrium; M, myometrium; ns, not significant.
Number of Pups Born From the Reciprocal Mating of C2αfl/fl;C2βfl/fl and C2αfl/fl;C2βfl/fl;SM22α-Cre Mice
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl | 42 (4) | 10.50 ± 0.29 |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl;SM22α-Cre | 72 (7) | 10.29 ± 0.87 |
| C2αfl/fl;C2βfl/fl;SM22α-Cre | C2αfl/fl;C2βfl/fl | 28 (4) | 7.00 ± 0.71a |
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl | 42 (4) | 10.50 ± 0.29 |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl;SM22α-Cre | 72 (7) | 10.29 ± 0.87 |
| C2αfl/fl;C2βfl/fl;SM22α-Cre | C2αfl/fl;C2βfl/fl | 28 (4) | 7.00 ± 0.71a |
Values represent mean ± SEM.
P < 0.05, significantly different from the matings of C2αfl/fl;C2βfl/fl (female) × C2αfl/fl;C2βfl/fl (male) mice and C2αfl/fl;C2βfl/fl (female) × C2αfl/fl;C2βfl/fl ;SM22α-Cre (male) mice.
Number of Pups Born From the Reciprocal Mating of C2αfl/fl;C2βfl/fl and C2αfl/fl;C2βfl/fl;SM22α-Cre Mice
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl | 42 (4) | 10.50 ± 0.29 |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl;SM22α-Cre | 72 (7) | 10.29 ± 0.87 |
| C2αfl/fl;C2βfl/fl;SM22α-Cre | C2αfl/fl;C2βfl/fl | 28 (4) | 7.00 ± 0.71a |
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl | 42 (4) | 10.50 ± 0.29 |
| C2αfl/fl;C2βfl/fl | C2αfl/fl;C2βfl/fl;SM22α-Cre | 72 (7) | 10.29 ± 0.87 |
| C2αfl/fl;C2βfl/fl;SM22α-Cre | C2αfl/fl;C2βfl/fl | 28 (4) | 7.00 ± 0.71a |
Values represent mean ± SEM.
P < 0.05, significantly different from the matings of C2αfl/fl;C2βfl/fl (female) × C2αfl/fl;C2βfl/fl (male) mice and C2αfl/fl;C2βfl/fl (female) × C2αfl/fl;C2βfl/fl ;SM22α-Cre (male) mice.
Number of Pups Born From the Reciprocal Mating of C2αfl/fl;C2β−/− and C2αfl/fl;C2β−/−;SM22α-Cre Mice
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2β−/− | C2αfl/fl;C2β−/−;SM22α-Cre | 83 (9) | 9.22 ± 0.40 |
| C2αfl/fl;C2β−/−;SM22α-Cre | C2αfl/fl;C2β−/− | 40 (6) | 6.67 ± 0.56a |
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2β−/− | C2αfl/fl;C2β−/−;SM22α-Cre | 83 (9) | 9.22 ± 0.40 |
| C2αfl/fl;C2β−/−;SM22α-Cre | C2αfl/fl;C2β−/− | 40 (6) | 6.67 ± 0.56a |
Values represent mean ± SEM.
P < 0.01, significantly different from the mating of C2αfl/fl;C2β−/− (female) × C2αfl/fl;C2β−/−;SM22α-Cre (male) mice.
Number of Pups Born From the Reciprocal Mating of C2αfl/fl;C2β−/− and C2αfl/fl;C2β−/−;SM22α-Cre Mice
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2β−/− | C2αfl/fl;C2β−/−;SM22α-Cre | 83 (9) | 9.22 ± 0.40 |
| C2αfl/fl;C2β−/−;SM22α-Cre | C2αfl/fl;C2β−/− | 40 (6) | 6.67 ± 0.56a |
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2αfl/fl;C2β−/− | C2αfl/fl;C2β−/−;SM22α-Cre | 83 (9) | 9.22 ± 0.40 |
| C2αfl/fl;C2β−/−;SM22α-Cre | C2αfl/fl;C2β−/− | 40 (6) | 6.67 ± 0.56a |
Values represent mean ± SEM.
P < 0.01, significantly different from the mating of C2αfl/fl;C2β−/− (female) × C2αfl/fl;C2β−/−;SM22α-Cre (male) mice.
Number of Pups Born From the Reciprocal Mating of Female and Male Mice With Indicated Genotypes
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2α+/+;C2β+/+ (wild-type) | C2α+/+;C2β+/+ (wild-type) | 68 (8) | 8.50 ± 0.33 |
| C2αfl/fl | C2αfl/fl;SM22α-Cre | 34 (4) | 8.50 ± 0.87 |
| C2αfl/fl;SM22α-Cre | C2αfl/fl | 30 (4) | 7.50 ± 0.65 |
| C2β3lox/3lox | C2β3lox/3lox;SM22α-Cre | 23 (3) | 7.67 ± 0.33 |
| C2β3lox/3lox;SM22α-Cre | C2β3lox/3lox | 25 (3) | 8.33 ± 0.67 |
| C2αfl/fl;C2β3lox/3lox | C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | 104 (12) | 8.67 ± 0.26 |
| C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | C2αfl/fl;C2β3lox/3lox | 68 (11) | 6.18 ± 0.54a,b |
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2α+/+;C2β+/+ (wild-type) | C2α+/+;C2β+/+ (wild-type) | 68 (8) | 8.50 ± 0.33 |
| C2αfl/fl | C2αfl/fl;SM22α-Cre | 34 (4) | 8.50 ± 0.87 |
| C2αfl/fl;SM22α-Cre | C2αfl/fl | 30 (4) | 7.50 ± 0.65 |
| C2β3lox/3lox | C2β3lox/3lox;SM22α-Cre | 23 (3) | 7.67 ± 0.33 |
| C2β3lox/3lox;SM22α-Cre | C2β3lox/3lox | 25 (3) | 8.33 ± 0.67 |
| C2αfl/fl;C2β3lox/3lox | C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | 104 (12) | 8.67 ± 0.26 |
| C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | C2αfl/fl;C2β3lox/3lox | 68 (11) | 6.18 ± 0.54a,b |
Values represent mean ± SEM.
P < 0.05, significantly different from the mating of C2α+/+;C2β+/+ (female) × C2α+/+;C2β+/+ (male) mice.
P < 0.01, significantly different from the mating of C2αfl/fl;C2β3lox/3lox (female) × C2αfl/fl;C2β3lox/3lox;SM22α-Cre (male) mice.
Number of Pups Born From the Reciprocal Mating of Female and Male Mice With Indicated Genotypes
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2α+/+;C2β+/+ (wild-type) | C2α+/+;C2β+/+ (wild-type) | 68 (8) | 8.50 ± 0.33 |
| C2αfl/fl | C2αfl/fl;SM22α-Cre | 34 (4) | 8.50 ± 0.87 |
| C2αfl/fl;SM22α-Cre | C2αfl/fl | 30 (4) | 7.50 ± 0.65 |
| C2β3lox/3lox | C2β3lox/3lox;SM22α-Cre | 23 (3) | 7.67 ± 0.33 |
| C2β3lox/3lox;SM22α-Cre | C2β3lox/3lox | 25 (3) | 8.33 ± 0.67 |
| C2αfl/fl;C2β3lox/3lox | C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | 104 (12) | 8.67 ± 0.26 |
| C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | C2αfl/fl;C2β3lox/3lox | 68 (11) | 6.18 ± 0.54a,b |
| Mating Strategy (Female × Male) . | . | ||
|---|---|---|---|
| Female Genotype . | Male Genotype . | Pups (Litters) . | Average Litter Size . |
| C2α+/+;C2β+/+ (wild-type) | C2α+/+;C2β+/+ (wild-type) | 68 (8) | 8.50 ± 0.33 |
| C2αfl/fl | C2αfl/fl;SM22α-Cre | 34 (4) | 8.50 ± 0.87 |
| C2αfl/fl;SM22α-Cre | C2αfl/fl | 30 (4) | 7.50 ± 0.65 |
| C2β3lox/3lox | C2β3lox/3lox;SM22α-Cre | 23 (3) | 7.67 ± 0.33 |
| C2β3lox/3lox;SM22α-Cre | C2β3lox/3lox | 25 (3) | 8.33 ± 0.67 |
| C2αfl/fl;C2β3lox/3lox | C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | 104 (12) | 8.67 ± 0.26 |
| C2αfl/fl ;C2β3lox/3lox ;SM22α-Cre | C2αfl/fl;C2β3lox/3lox | 68 (11) | 6.18 ± 0.54a,b |
Values represent mean ± SEM.
P < 0.05, significantly different from the mating of C2α+/+;C2β+/+ (female) × C2α+/+;C2β+/+ (male) mice.
P < 0.01, significantly different from the mating of C2αfl/fl;C2β3lox/3lox (female) × C2αfl/fl;C2β3lox/3lox;SM22α-Cre (male) mice.
Contraction of uterine smooth muscle from smDKO mice is impaired
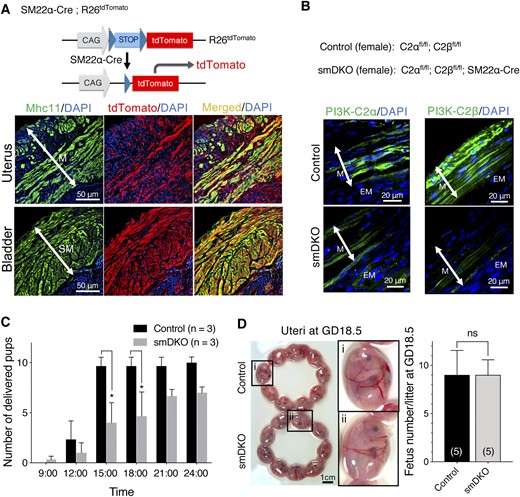
The gross structure of the uterus was similar in female control and smDKO mice. The myometrium in the uterus of smDKO mice showed the well-developed outer longitudinal and inner circular layers with normal thickness compared with control C2αfl/fl;C2βfl/fl mice (Fig. 2A and 2B). The endometrium in smDKO mice also appeared normal microscopically compared with control C2αfl/fl;C2βfl/fl mice (Fig. 2B). Additionally, the expression levels of different contractile and smooth muscle–specific proteins, including smooth muscle–specific Mhc11, MLCK, the actin filament-associated caldesmon and calponin, the catalytic subunit of MLC20 phosphatase (MLCP) PP1δ, and the myosin-binding regulatory subunit of MLCP MYPT1, were all similar in the uteri of control and smDKO mice at both the nonpregnant and pregnant (GD18.5) stages (Fig. 2C). The expression of C2α protein in the myometrium of control mice was also similar at the nonpregnant and pregnant (GD18.5) stages (Fig. 2D). C2β protein in pregnant control mice tended to be lower compared with nonpregnant control mice. The expression of C2α and C2β in smDKO mice was substantially reduced at the nonpregnant and pregnant stages compared with control mice.
No abnormality in uterine morphology and smooth muscle–specific protein expression in control and C2α and C2β DKO mice. (A) Gross views of the uteri of control and smDKO nonpregnant mice. (B) Hematoxylin and eosin staining of uterine sections of control and smDKO nonpregnant mice and myometrial thickness of control and smDKO mice. The representative histological images (left) and quantified data (right) are shown. (C) Expression of various smooth muscle–specific proteins in the myometrial tissues of nonpregnant and pregnant (GD18.5) control and smDKO mice (top). The representative western blots (top) and quantified data (bottom) are shown. (D) Expression of C2α and C2β proteins in the myometrial tissues of nonpregnant and pregnant (GD18.5) control and smDKO mice (top). The representative western blots (top) and quantified data (bottom) are shown. In (B) and (D), the numbers in the columns denote the numbers of analyzed mice. The data in (B)–(D) are expressed as means ± SEM. *P < 0.05; **P < 0.01. ns, not significant.
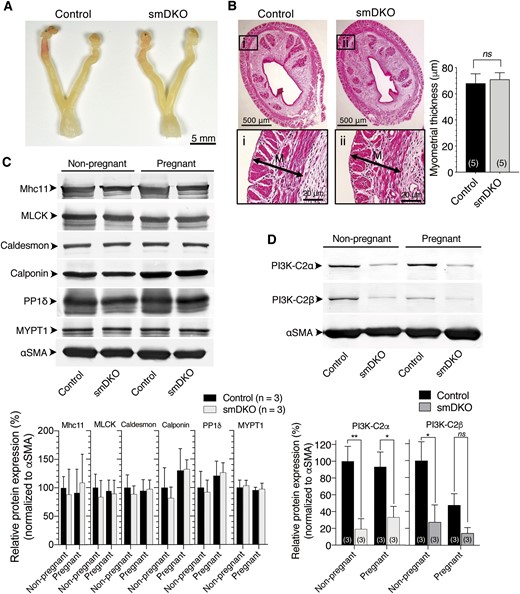
We next studied the possibility that smDKO pregnant mice might have impaired uterine contraction, which led to the impeded delivery. We prepared uterine strips from control and KO mice at GD18.5 and compared contractile responses. The uterine strips showed spontaneous, repeated contraction of the duration of several seconds under the isometric condition. The amplitude and frequency of spontaneous contraction were both reduced in uterine strips from smDKO mice compared with those from control mice (Fig. 3A). The uterine strips from single C2α KO (smC2αKO) mice showed similar amplitudes and frequency of spontaneous contraction to those of control mouse strips. The uterine strips from single C2β KO (smC2βKO) mice showed similar amplitude but lower frequency of spontaneous contraction compared with control mice. Similar to the case of spontaneous contraction, the amplitudes of contractile responses induced by oxytocin and KCl, which activate phospholipase C/Rho pathways and the L-type voltage-dependent Ca2+ channel, respectively, were also diminished in smDKO but not smC2αKO and smC2βKO uterine strips compared with control mice (Fig. 3B and 3C). Oxytocin and KCl increased spike frequency to similar extents in control, smC2αKO, smC2βKO, and smDKO strips (lower panels of Fig. 3B and 3C), compared with oxytocin- and KCl-nonstimulated strips (lower panel of Fig. 3A).
Diminished contractile responses with reduced Rho kinase dependence in USM of C2α and C2β DKO mice. The isometric tension was determined in the uterine strips from control, single smKO (smC2αKO and smC2βKO) mice, and smDKO mice. Amplitudes and frequencies of (A) spontaneous contraction, (B) KCl-induced contraction, and (C) oxytocin-induced contraction. The uterine strips were stimulated with 200 nM oxytocin or 60 mM KCl or unstimulated, and maximal tensions and spike frequencies were determined. (D) Reductions of contraction by the L-type Ca2+ channel antagonist nitrendipine (NTD). The amplitudes and frequency of spontaneous contraction and amplitudes of oxytocin-induced contraction were determined with or without NTD (100 nM) pretreatment as described in “Materials and Methods.” (E) Reductions of contraction by the Rho kinase inhibitor Y27632. The amplitudes and frequency of spontaneous contraction and amplitudes of oxytocin-induced contraction were determined with or without Y27632 (10 μM) pretreatment as described in “Materials and Methods.” In (A)–(E), the numbers in the columns denote the numbers of analyzed strips. The data are expressed as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
To explore the mechanisms by which uterine contraction is dependent on C2α and C2β, we compared the responses of uterine contraction to the L-type voltage-dependent Ca2+ channel antagonist nitrendipine and the Rho kinase inhibitor Y-27632 in control and smDKO strips. Nitrendipine substantially inhibited spontaneous contraction in both control and smDKO strips. Nitrendipine relatively weakly inhibited oxytocin-induced contraction in control and smDKO strips compared with spontaneous contraction. These observations suggest that both L-type Ca2+ channel–dependent and –independent mechanisms are involved in spontaneous and oxytocin-induced contraction in control and smDKO USM. The amplitudes of spontaneous and oxytocin-induced contraction in the presence of nitrendipine were similar in control and smDKO strips (Fig. 3D). These results may suggest that L-type Ca2+ channel–dependent contraction involves both C2α/C2β-dependent and -independent contractile mechanisms whereas L-type Ca2+ channel–independent contraction does not require C2α or C2β. Y-27632 partially inhibited spontaneous- and oxytocin-induced contraction in control strips, but it did not significantly inhibit contraction of smDKO strips (Fig. 3E), suggesting that both Rho kinase–dependent and –independent contractile mechanisms are involved in spontaneous and oxytocin-induced contraction in control strips. In contrast, contraction of smDKO USM is largely Rho kinase–independent. It is suggested that Rho kinase–dependent contraction requires C2α and C2β whereas Rho kinase–independent contraction does not.
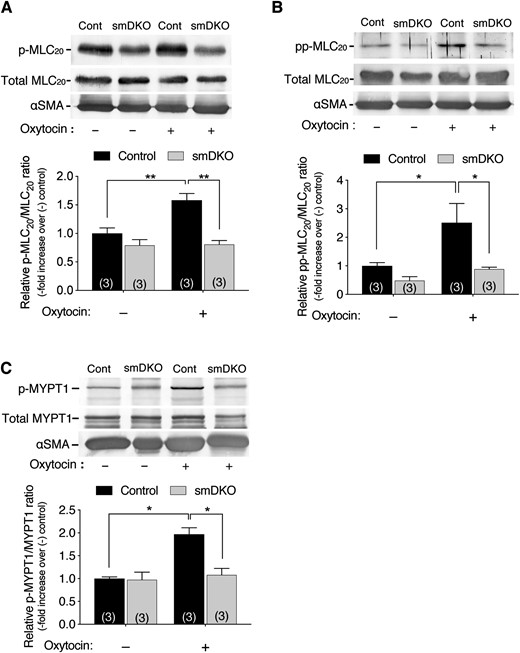
Oxytocin-induced phosphorylation of MLC20 and MYPT1 is attenuated in uterine smooth muscle tissues of smDKO mice
We compared oxytocin-induced phosphorylation of MLC20 and MYPT1, the latter phosphorylation of which inhibits MLCP to lead to an increase in MLC20 phosphorylation, in uterine strips of smDKO and control mice. Oxytocin induced a 1.6-fold increase in monophosphorylation (Ser19) of MLC20 in uterine strips of control mice, but not in those of smDKO mice (Fig. 4A). Oxytocin also induced a 2.6-fold increase in diphosphorylation (Thr18/Ser19) of MLC20, which is known to be increased when MLCP is inhibited or MLCK activity becomes very high (27), in control mouse uterine strips (Fig. 4B). In contrast, in smDKO mouse strips, oxytocin did not increase the diphosphorylation (Thr18/Ser19) of MLC20 level above that in the nonstimulated control strips. Furthermore, oxytocin induced a 2.0-fold increase in phosphorylation (Thr853) of the MLCP regulatory subunit MYPT1, which results in inhibition of MLCP, in control mouse strips but not in smDKO mouse strips (Fig. 4C). Thus, both monophosphorylation and diphosphorylation levels of MLC20 are reduced with diminished inhibition of MLCP in smDKO mouse strips.
Diminished oxytocin-induced phosphorylation of MLC20 and MYPT1 in USM of C2α and C2β DKO mice. (A–C) The USM strips isometrically contracted in response to oxytocin (200 nM) stimulation were snap frozen and analyzed for phosphorylation of MLC20 at Ser19 (p-MLC20) (A) and at Thr18/Ser19 (pp-MLC20) (B), as well as MYPT1 (p-MYPT1) at Thr853 (C). The USM strips were frozen at 3, 10, and 10 min, respectively, after oxytocin addition for the determinations of p-MLC20, pp-MLC20, and p-MYPT1. In (A)–(C), the numbers in the columns denote the numbers of analyzed samples. The data are expressed as means ± SEM. *P < 0.05; **P < 0.01.
Rho signaling, but not Ca2+ signaling, is downregulated in smDKO myometrial cells
We isolated myometrial cells from control and smDKO pregnant mice. The morphology of the isolated cells observed under bright field microscopy was similar between control and smDKO mice (Fig. 5A). We identified 70% of the isolated cells as smooth muscle, using mice that expressed the tdTomato reporter gene specifically in smooth muscle (Fig. 5B).
Diminished oxytocin-induced Rho activation, but not intracellular Ca2+ mobilization or Ca2+ channel protein expression, in myometrial smooth muscle cells from C2α and C2β DKO mice. Myometrial smooth muscle cells were isolated from control and DKO mice. (A) Phase-contrast images of myometrial smooth muscle cells. (B) Smooth muscle–specific expression of tdTomato fluorescent protein in mice that carries the R26-tdTomato reporter construct. Nuclei were stained with DAPI. (C) Oxytocin-induced increase in the [Ca2+]i in USM cells from control and smDKO mice. Cells were stimulated with 100 nM oxytocin. The quantified data show the [Ca2+]i peak response from 21 control and 20 smDKO cells. (D) Western blotting of L-type Ca2+ channel protein Cavα2 in USM cells from control and smDKO mice. (E) Immunofluorescent staining of the Ca2+ channel Cavα2 and the K+ channel BKα1 in the myometrium of control and smDKO mice. The boxed regions are shown as the magnified views obtained with SRRF microscopy. The red arrowheads denote Cavα2 protein of the L-type Ca2+ channel in the first SRRF view panel (left) and BKα1 protein of K+ channel of the last SRRF view panel (right). (F) FRET imaging of Rho activation in USM cells from control and smDKO mice. Left, representative images of Rho-FRET signals. Right, quantified data from nine control and nine smDKO cells. The peak/basal signal ratio in control cells was expressed as 100%. Red arrowhead denotes Rho activation signal. In (C), (D), and (F), the numbers in the columns denote the numbers of analyzed samples. The data in (C), (D), and (F) are expressed as means ± SEM. ***P < 0.001. M, myometrial layer of uterus; ns, not significant; Oxy, oxytocin.
We determined oxytocin-induced changes in the [Ca2+]i in isolated myometrial smooth muscle. Oxytocin induced similar extents (approximately sixfold) of the peak increases in the [Ca2+]i in both control and smDKO myometrial smooth muscle (Fig. 5C). Because USM contraction is dependent on L-type Ca2+ channels, we analyzed the protein expression of Cavα2 in control and smDKO mice uterus. The expression level of Cavα2 protein in the uterus was similar in control and smDKO mice as evaluated by western blotting (Fig. 5D). Immunofluorescence of the uterine tissues with anti-Cavα2 antibody showed that the myometrial smooth muscle in both control and smDKO uterus expressed this protein (Fig. 5E, left). With SRRF microscopy, we obtained higher resolution views of the immunostained myometrial layer, which showed that Cavα2 was distributed mainly on the plasma membrane or its vicinity of smooth muscle (Fig. 5E, left). The BK channel, which is a negative regulator of l-type calcium channels, was also distributed largely on the plasma membrane or its vicinity of smooth muscle, and there was no detectable difference of BK channel expression in control and smDKO mouse uterus (Fig. 5E, right).
The phosphorylation of MYPT1, which is controlled mainly by Rho kinase downstream of Rho, was decreased in smDKO USM (Fig. 4C). Therefore, we determined oxytocin-induced Rho activation in isolated myometrial smooth muscle cells, using a FRET imaging technique. In control smooth muscle cells, we observed substantial oxytocin-induced Rho activation signals at the plasma membrane and the intracellular compartment. In smDKO myometrial smooth muscle cells, Rho activation signals at both the plasma membrane and the intracellular compartment were diminished (Fig. 5F).
Discussion
In parturition, the USM develops potent contractile force to expel the fetus. Dramatic changes in the signaling and contractile machineries of USM as well as the neuroendocrine functions at the end of pregnancy allow generation of potent uterine contraction at parturition (1, 2, 28, 29). In the current study, we identified the novel smooth muscle molecules, C2α and C2β, which are required for full contraction of USM and normal delivery. C2α and C2β are involved in activation of Rho and resultant Rho kinase–dependent inhibition of MLCP, which potentiates MLC20 phosphorylation and contractile force in USM (27–31). Highly homologous C2α and C2β completely compensate for the effects of single gene deletion of each other in parturition, and only DKO results in defects in parturition.
In USM, activation of cell surface oxytocin and prostaglandin F2α receptors and Ca2+ channels, which include the L-type voltage-dependent Ca2+ channels, triggers an increase in [Ca2+]i by Ca2+ release from the intracellular Ca2+ store and Ca2+ entry through the plasma membrane Ca2+ channels (28, 30, 32). The increase in the [Ca2+]i activates Ca2+/calmodulin-dependent MLCK (Ca2+–MLCK pathway), leading to MLC20 phosphorylation and smooth muscle contraction. Oxytocin and prostaglandin F2α also activate small GTPase Rho via the heterotrimeric G protein G12/13 (28, 30, 31). Rho activates its effector Rho kinase, resulting in inhibition of MLCP by phosphorylating the regulatory subunit of MLCP, MYPT1 (Rho–Rho kinase–MLCP pathway) (27). Inhibition of MLCP potentiates MLCK-catalyzed MLC20 phosphorylation and contraction. Therefore, the Rho–MLCP pathway as well as the Ca2+–MLCK pathway coordinately and effectively increase MLC20 phosphorylation, thus playing a critical role in oxytocin-induced USM contraction (28–31, 33, 34). The Ca2+ signaling pathway and Rho–Rho kinase signaling pathways may have cross-communication because it was reported that Ca2+ entry across the plasma membrane activated Rho and Rho kinase in several different types of smooth muscle and that Rho kinase activated voltage-dependent Ca2+ channels through phosphorylation (22, 23, 35). Importantly, the USM in the late pregnancy has increased oxytocin receptor number and depolarization of the resting membrane potential, which increase excitability and contraction of USM (28, 29, 31–34).
The contraction data in the current study show that not only oxytocin-induced contraction but also spontaneous and KCl membrane depolarization-induced contraction was attenuated in smDKO USM compared with control smooth muscle. Mechanistically, spontaneous and membrane depolarization-induced contraction as well as oxytocin-induced contraction were inhibited by a Rho kinase inhibitor, suggesting the contribution of the Rho kinase pathway to these contractile responses in control mice. Notably, the extents of contraction inhibition by a Rho kinase inhibitor were marginal in smDKO USM, suggesting that the contribution of the Rho kinase pathway to contraction was greatly diminished in smDKO smooth muscle. The decreased MYPT1 phosphorylation and MLC20 diphosphorylation in oxytocin-stimulated smooth muscle supported this notion (27). The direct measurements of Rho activation in the myometrial cells by the FRET imaging technique showed that oxytocin-induced Rho activation was decreased in smDKO myometrial cells. Thus, it is likely that the decreased Rho activity results in a higher MLCP activity, leading to reductions in MLC20 phosphorylation and contraction in smDKO smooth muscle. These data also suggest in smDKO smooth muscle that decreased Rho kinase activity might induce diminished activation of l-type Ca2+ channels and, thereby, reduced spontaneous and KCl-induced contraction (35).
In our previous study (14), we found that VEGF-induced Rho activation occurred in the intracellular endosomal compartment, in which the internalized VEGF receptor-2 resided in endothelial cells. Both processes of the VEGF receptor-2 internalization and endosomal Rho activation required C2α. We also observed that C2α is required for the endosomal signaling induced by other receptor ligands, including S1P and TGFβ1 (9, 15). The catalytic product of C2α, PI(3,4)P2, contributes to endocytosis by recruiting various PI(3,4)P2-binding domain-containing proteins, which include SNX9, and promoting clathrin-coated vesicle formation (36). A role of C2β in endocytosis is currently almost unknown. Because C2α single KO did not impair USM contraction, very likely C2β can compensate for a defect caused by C2α deficiency in USM. In smDKO USM, the amount of PI(3,4)P2 produced by slightly expressed C2α and C2β is probably insufficient for receptor internalization and subsequent signaling in the endosomes in which receptors and their associated signaling molecules are assembled. A special form of Rho–guanine nucleotide exchange factor (GEF) is implicated in Rho activation in the endosomes (37, 38). The Rho-GEF Syx, which was found to be involved in VEGF-induced regulation of cell junctions, is one of the candidate Rho-GEFs. Further studies are required for identifying a Rho-GEF involved in endosomal Rho activation and clarifying the mechanism of recruitment and activation of a Rho-GEF.
Because the RhoA–Rho kinase–MLCP pathway is a major contractile mechanism in spontaneous and uterotonin-induced contraction (28), the new role of these two class II PI3K isoforms in the Rho–Rho kinase–MLCP pathway may provide some insight about the mechanisms for clinical conditions of abnormal uterine contraction, including preterm labor, uterine inertia, and life-threatening postpartum hemorrhage. For preterm labor, tocolytic agents are administered (39). Administration of class II PI3K inhibitors, which inhibit Rho and thereby stimulate MLCP in USM, may be candidates for developing new tocolytic agents. On the contrary, for clinical conditions of insufficient uterine contraction such as uterine inertia and life-threatening postpartum hemorrhage, stimulators of class II PI3Ks may bring about beneficial outcome by increasing Rho activity and thereby inhibiting MLCP activity with augmented uterine contraction. The inhibitory and stimulatory mechanisms of C2α and C2β at cellular levels are currently not well understood. Therefore, further studies are required to unravel the regulatory mechanism of human class II PI3K activity.
In summary, our study shows the importance of the novel molecules C2α and C2β in the regulation of the Rho–Rho kinase–MLCP pathway and their requirement for full contraction of USM and parturition. It is possible that dysfunctions of class II PI3K might lead to clinical conditions caused by abnormal USM contraction. Further studies are required to explore the detailed mechanisms of class II PI3K actions and the regulation of their activity, which will provide further insight into the physiological and pathophysiological roles of class II PI3Ks.
Abbreviations:
- C2α
phosphoinositide 3-kinase–C2α
- C2β
phosphoinositide 3-kinase–C2α
- [Ca2+]i
intracellular free Ca2+ concentration
- Cavα2
calcium channel L-type DHPR α subunit
- CFP
cyan fluorescent protein
- Cre
Cre recombinase
- DAPI
4′,6-diamidino-2-phenylindole
- DKO
double knockout
- FRET
fluorescence resonance energy transfer
- GD
gestational day
- GEF
guanine nucleotide exchange factor
- HBSS
Hanks' balanced salt solution
- KO
knockout
- Mhc11
myosin heavy chain 11
- MLC
myosin light chain
- MLC20
20-kDa myosin light chain
- MLCK
myosin light chain kinase
- MLCP
20-kDa myosin light chain phosphatase
- MYPT1
myosin phosphatase target subunit-1
- PI3K
phosphoinositide 3-kinase
- PI3K-C2
class II phosphoinositide 3-kinase
- PI(3,4)P2
phosphatidylinositol 3,4-bisphosphate
- R26-tdTomato
Rosa26-CAG-loxP-stop-loxP-tdTomato
- smC2αKO
smooth muscle–specific knockout of C2α
- smC2βKO
smooth muscle–specific knockout of C2β
- smDKO
smooth muscle–specific double knockout
- S1P
sphingosine-1-phosphate
- SRRF
super-resolution radial fluctuation
- USM
uterine smooth muscle
- VEGF
vascular endothelial growth factor
- YFP
yellow fluorescent protein
Acknowledgments
We thank Chiemi Hirose for secretarial assistance.
Financial Support: This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grant 25116711 (to Y.T.) and by Japan Society for the Promotion of Science Grants 17K08532 (to K.Y.), 16K18988 (to S.A.), 17K08542 (to N.T.), and 15H04673 (to Y.T.).
Disclosure Summary: The authors have nothing to disclose.


![Diminished oxytocin-induced Rho activation, but not intracellular Ca2+ mobilization or Ca2+ channel protein expression, in myometrial smooth muscle cells from C2α and C2β DKO mice. Myometrial smooth muscle cells were isolated from control and DKO mice. (A) Phase-contrast images of myometrial smooth muscle cells. (B) Smooth muscle–specific expression of tdTomato fluorescent protein in mice that carries the R26-tdTomato reporter construct. Nuclei were stained with DAPI. (C) Oxytocin-induced increase in the [Ca2+]i in USM cells from control and smDKO mice. Cells were stimulated with 100 nM oxytocin. The quantified data show the [Ca2+]i peak response from 21 control and 20 smDKO cells. (D) Western blotting of L-type Ca2+ channel protein Cavα2 in USM cells from control and smDKO mice. (E) Immunofluorescent staining of the Ca2+ channel Cavα2 and the K+ channel BKα1 in the myometrium of control and smDKO mice. The boxed regions are shown as the magnified views obtained with SRRF microscopy. The red arrowheads denote Cavα2 protein of the L-type Ca2+ channel in the first SRRF view panel (left) and BKα1 protein of K+ channel of the last SRRF view panel (right). (F) FRET imaging of Rho activation in USM cells from control and smDKO mice. Left, representative images of Rho-FRET signals. Right, quantified data from nine control and nine smDKO cells. The peak/basal signal ratio in control cells was expressed as 100%. Red arrowhead denotes Rho activation signal. In (C), (D), and (F), the numbers in the columns denote the numbers of analyzed samples. The data in (C), (D), and (F) are expressed as means ± SEM. ***P < 0.001. M, myometrial layer of uterus; ns, not significant; Oxy, oxytocin.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/endo/160/1/10.1210_en.2018-00756/1/m_en.2018-00756f5.jpeg?Expires=1716404849&Signature=WBPHFuz37IpfRFa6FX7qRuitONfvzTcc2r0qSxErPbjsLVp8oSdUrgG58-gFjP~Mv0nxVw3fnuMST3~pEuI1IU0C~fIV9pV9EOiRYyDoluwSwtXelPNxkPG~nhP5GD9lBmR6o6Ngs2fepbRdfT4PTTdl~GtBwnEkg3D7-5~8AHH~dEzQD0M3-ajiuE9vqDCK3K1FTk9KfhGb1JvK4neCVTUQi8B~I3GfRdfEiHUjuuYNyPy9UXRyieVWsZHLr0mIChOrbKS5QIS9HBhj8PcVAhElB9RNjrlf50hTiUwIKh4iMBPP2wMsZeMpKP0N~oEHe1nzLfR2D8MUmdax6xzYsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
