-
PDF
- Split View
-
Views
-
Cite
Cite
C. Gillet, D. Spruyt, S. Rigutto, A. Dalla Valle, J. Berlier, C. Louis, C. Debier, N. Gaspard, W. J. Malaisse, V. Gangji, J. Rasschaert, Oleate Abrogates Palmitate-Induced Lipotoxicity and Proinflammatory Response in Human Bone Marrow-Derived Mesenchymal Stem Cells and Osteoblastic Cells, Endocrinology, Volume 156, Issue 11, 1 November 2015, Pages 4081–4093, https://doi.org/10.1210/en.2015-1303
Close - Share Icon Share
Osteoporosis is a metabolic bone disease associated with unequilibrated bone remodeling resulting from decreased bone formation and/or increased bone resorption, leading to progressive bone loss. In osteoporotic patients, low bone mass is associated with an increase of bone marrow fat resulting from accumulation of adipocytes within the bone marrow. Marrow adipocytes are active secretory cells, releasing cytokines, adipokines and free fatty acids (FA) that influence the bone marrow microenvironment and alter the biology of neighboring cells. Therefore, we examined the effect of palmitate (Palm) and oleate (Ole), 2 highly prevalent FA in human organism and diet, on the function and survival of human mesenchymal stem cells (MSC) and MSC-derived osteoblastic cells. The saturated FA Palm exerted a cytotoxic action via initiation of endoplasmic reticulum stress and activation of the nuclear factor κB (NF-κB) and ERK pathways. In addition, Palm induced a proinflammatory response, as determined by the up-regulation of Toll-like receptor 4 expression as well as the increase of IL-6 and IL-8 expression and secretion. Moreover, we showed that MSC-derived osteoblastic cells were more sensitive to lipotoxicity than undifferentiated MSC. The monounsaturated FA Ole fully neutralized Palm-induced lipotoxicity by impairing activation of the pathways triggered by the saturated FA. Moreover, Ole promoted Palm detoxification by fostering its esterification into triglycerides and storage in lipid droplets. Altogether, our data showed that physiological concentrations of Palm and Ole differently modulated cell death and function in bone cells. We therefore propose that FA could influence skeletal health.
Osteoporosis is a metabolic bone disease prevalent in postmenopausal women and the elderly, associated with unequilibrated bone remodeling resulting from decreased bone formation and/or accelerated bone resorption. As a result, the refilling of resorption cavities can be incomplete, leading to progressive bone loss, increased bone fragility, and fracture risk (1).
Bone formation is fulfilled by osteoblastic cells (Ob) originating from the bone marrow-derived mesenchymal stem cells (MSC). MSC are pluripotent progenitor cells able to differentiate in Ob and other cell types such as adipocytes and chondrocytes (2). Commitment of MSC to the osteoblast lineage is controlled by growth factors, hormones, and cytokines and is sensitive to environmental conditions (3). In osteoporotic patients, the number of MSC is reduced and is correlated with an increased apoptosis of MSC and osteocytes (4). Moreover, osteoporosis is associated with adipocytes accumulation in the bone marrow niche due to a preferential differentiation of MSC toward adipocytes at the expense of osteoblast formation (5, 6). Of note, a rise in bone marrow adiposity is also observed in ageing and, particularly, in secondary osteoporosis associated with glucocorticoids therapy or type 2 diabetes mellitus treatments such as peroxisome proliferator-activated receptor-γ agonists (7–9).
Adipocytes are integral components of the bone marrow, but their physiological function in the bone marrow stem cell niche as well as the consequence of their excessive accumulation in pathological entities remain elusive (10). Although they have long been considered as inert cells, it is now widely accepted that adipocytes constitute a metabolically active depot that may affect skeletal metabolism (11–14). By releasing factors such as fatty acids (FA), adipokines, and cytokines, marrow adipocytes may generate a local microenvironment that could disturb bone cells viability and function (13, 15, 16). If the impact of cytokines and adipokines on bone health has been assessed, the effects of FA on the biological function and survival of MSC and Ob have not yet been properly studied.
In physiological conditions, FA serve as key sources of cellular metabolic fuel and an acute exposure to these nutrients is usually beneficial for mammalian cells. Yet, a chronic exposure to FA may lead to ectopic lipid deposition in cells of nonadipose tissue. This unsuited FA accumulation is now increasingly recognized to contribute to organ injury through a process termed lipotoxicity (17). Sensitivity to lipotoxicity differs across cell types. In myocytes, hepatocytes and insulin-producing pancreatic β-cells, excessive FA availability results in cellular dysfunction and death (18–20), leading to disease states (17). If the contribution to lipotoxicity of long-chain saturated and unsaturated FA varies depending on the cell type, FA toxicity is, however, often associated with the saturated ones (21).
We postulated that, in the setting of osteoporosis, excessive adipocytes accumulation in the bone marrow niche may favor the local release of larger quantities of saturated FA which could interfere with bone cells homeostasis. This hypothesis is supported by both in vitro and in vivo observations. Human primary coculture experiments of adipocytes originating from mammary adipose tissue and MSC-derived osteoblasts have shown that adipocytes liberate factors that inhibit preosteoblast proliferation and differentiation (22, 23). In a similar experimental model, MSC-derived adipocytes enhanced Ob apoptosis via the release of saturated FA (24, 25). Recently, the study of Yeung et al demonstrated that the higher marrow fat content observed in osteoporotic subjects is characterized by an increased ratio of saturated vs unsaturated FA (26). Moreover, in diabetic patients, the proportion of saturated vs unsaturated FA in the marrow fat was associated with modifications of the fracture risk (27). Additionally, it is well known that the FA composition of the diet (saturated, mono- or polyunsaturated) influences the FA profile of the triglycerides (TG) stored in adipocytes. Indeed, a higher consumption of saturated FA is negatively associated with bone mineral density (28) and is linked to an increased risk of osteoporotic fractures (29).
The purpose of this study was therefore to examine the influence of FA on the function and survival of human MSC and Ob. We selected 2 FA widely represented in human blood and diet (30): palmitate (Palm), a saturated FA (C16:0), and oleate (Ole), a monounsaturated one (C18:1 n-9). Palm and Ole have been extensively used in previous studies to evaluate the effects of FA on various cell types (31–33).
Our data showed that physiological concentrations of the 2 FA differently regulated cell death and function.
Materials and Methods
Isolation, culture, and characterization of human MSC and MSC-derived Ob
Human MSC were obtained from bone marrow aspirated from the posterior iliac crest of healthy donors (18–50 y old). Our local institutional ethical committee approved the study and all subjects gave their written consent. Bone marrow was diluted 1:0.5 with PBS and overlaid on Ficoll-Paque PREMIUM (GE Healthcare). Mononuclear cells were isolated after centrifugation and seeded at a density of 5.7 × 104 cells/cm2 in DMEM low glucose (1 g/L; Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich), 100-U/mL penicillin, and 100-μg/mL streptomycin (Invitrogen). The culture medium was renewed after 4 days of culture and then every 2–3 days until cells reached confluence. MSC were detached by enzymatic treatment (trypsin-EDTA; Invitrogen) every week and successively seeded at a density of 5.7 × 103 cells/cm2 until passage 8.
To confirm the mesenchymal nature of the isolated cells, phenotyping by fluorescence-activated cell sorting and differentiation assays were performed. Cells were stained with the next antibodies: cluster of differentiation (CD) 34-phycoerythrin, CD45-fluorescein isothiocyanate (FITC), and CD73-PE from BD Biosciences, CD90-allophycocyanin (APC), CD105-FITC, and alkaline phosphatase (ALP)-APC from R&D Systems (34). We confirmed that the cells expressed CD73, CD90, and CD105 and were negative for CD34, CD45, and ALP (Supplemental Figure 1, A and B). Fluorescence was measured on a FACSCanto II flow cytometer (BD Biosciences) and data were processed using Diva software v5.0. At least 10 000 events were acquired for each sample. Supplemental validation showed that the cells had the capacity to differentiate in the osteoblastic and adipogenic lineage when cultured in standard in vitro differentiation assays (see below).
To obtain human MSC-derived Ob, MSC were cultured for 14 days in an osteogenic medium (DMEM low glucose containing 10% FBS, 100-U/mL penicillin, 100-μg/mL streptomycin, 10mM β-glycerophosphate (Sigma-Aldrich), 10nM dexamethasone (Sigma-Aldrich), and 50-μg/mL ascorbic acid (Sigma-Aldrich). The culture medium was renewed every 2 days. The osteogenic medium was then replaced by standard culture medium for 48 hours. Osteoblastic differentiation was validated before FA treatment by mineralization assay (alizarin red staining) and measurement of ALP, as previously described (Supplemental Figure 1C) (35, 36). Induction of the osteoblastic differentiation marker genes Runt-related transcription factor 2 (Runx2) and osteocalcin (OCN) was also confirmed by quantitative PCR (qPCR) (Supplemental Figure 1D).
For adipogenic differentiation, MSC were cultured for 14 days in an adipogenic medium (DMEM low glucose containing 10% FBS, 100-U/mL penicillin, 100-μg/mL streptomycin, 60μM indomethacin (Sigma-Aldrich), 0.5mM 3-isobutyl-1-methylxanthine (Gibco), 5-μg/mL insulin (Sigma-Aldrich), and 100nM dexamethasone (Sigma-Aldrich). The culture medium was renewed every 2 days. Adipogenesis was evaluated by Oil Red staining as previously described (Supplemental Figure 1C) (25).
SaOS-2 cells, a human Ob line (a kind gift from Bone Therapeutics) were grown in McCoy’s 5A medium (Invitrogen) supplemented with 10% FBS, 100-U/mL penicillin, and 100-μg/mL streptomycin.
Free FA treatment
Sodium Ole and sodium Palm (Sigma-Aldrich) were solubilized in 90% ethanol at 70°C to obtain 50mM stock solutions. For FA supplementation experiments, cells were cultured for the indicated times in a FA-specific medium composed of DMEM low glucose (for MSC) or McCoy’s 5A medium (for SaOS-2 cells) supplemented with 1% FBS, 100-U/mL penicillin, 100-μg/mL streptomycin, 1% FA free BSA (Sigma-Aldrich) and, when required, diluted Palm and/or Ole (0.125mM–0.50mM). Of note, a similar dilution of ethanol, the FA solvent, was added in the control condition (eg, absence of FA). Taking into account the stepwise equilibrium model and the respective binding affinities of Palm and Ole for BSA (37), the concentrations of FA used in our study are close to the physiological ones (30).
Determination of cell viability
Cells were seeded in 96-well plates at a density of 14.7 × 103 cells/cm2 for MSC and Ob, and 20.6 × 103 cells/cm2 for SaOS-2 cells. After 3 days, culture media were replaced by the FA-specific medium without or with increasing concentrations of Palm and/or Ole for the indicated times and concentrations. Cells were incubated for 15 minutes with the nuclear binding dyes propidium iodide (10 μg/mL) (Sigma-Aldrich) and Hoechst 33342 (10 μg/mL) (Sigma-Aldrich). Cell viability was examined by inverted microscopy (Axiovision Zeiss) with UV excitation (λ excitation/emission, 365/397 nm). Viable cells were characterized by their intact nuclei with blue fluorescence, whereas dead cells were depicted by yellow-red fluorescence. In each experimental condition, a minimum of 600 cells were counted.
Measurement of caspases-3/7 activity
Caspases-3/7 activity was determined using the Caspase-Glo 3/7 assay (Promega). 29.4 × 103 cells/cm2 were seeded in 96-well white plates. After 3 days, culture media were replaced by FA-specific medium supplemented with increasing concentrations of Palm, complemented or not with Ole, for the indicated times. At the end of the incubation period, 100 μL of culture medium were replaced by 100 μL of Caspase-Glo reagent resulting in cell lysis and cleavage of the Caspase-Glo 3/7 substrate by the activated caspases. The luminescent signal generated was detected after 1 hour using a Victor2 (PerkinElmer). Staurosporin 1μM (Sigma-Aldrich) was used as positive control.
Measurement of nuclear factor-κB (NF-κB) activation
SaOS-2 cells were plated in 8-chamber slides (BD Biosciences) at a density of 14.5 × 103 cells/cm2. After 3 days, culture media were replaced by FA-specific medium supplemented with Palm and/or Ole, for the indicated times. Cells treated for 1 hour with 30-UI/mL recombinant human IL-1β (R&D Systems) were used as positive control. To evaluate NF-κB nuclear translocation by immunocytochemistry, cells were fixed with 4% formaldehyde for 10 minutes and permeabilized with an acetone/methanol (7:3) mixture for 5 minutes. Nonspecific binding sites were blocked with 10% normal goat serum (Gibco) and 0.8% BSA fraction V (Sigma-Aldrich) for 30 minutes. Cells were then incubated overnight with rabbit polyclonal antibody raised against the p65 subunit of NF-κB (1:500; Santa Cruz Biotechnology, Inc). Finally, cells were incubated for 1 hour with donkey antirabbit IgG-FITC (1:200; Jackson ImmunoResearch), and nuclei were stained with Hoechst 33342 (Table 1). Cells were mounted using the Dako fluorescence mounting medium (Dako) and examined with inverted fluorescence microscope (Axio Zeiss).
Antibody Table
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| Phospho-p44/42 MAPK (Erk1/2) | (Thr202/Tyr204) | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell Signaling, 9101 | Polyclonal | 1:1000 |
| MAPK (Erk1/2) | / | Anti-MAPK (Erk1/2) | Millipore, 06-182 | Polyclonal | 1:1000 |
| Phospho-eIF2α | (Ser51) | Phospho-eIF2α (Ser51) (D9G8) rabbit mAb | Cell Signaling, 3398 | Monoclonal | 1:1000 |
| eIF2α | / | eIF2α (D7D3) rabbit mAb | Cell Signaling, 5324 | Monoclonal | 1:1000 |
| NF-κB | / | NF-κB p65 (C-20) | Santa Cruz Biotechnology, Inc, sc-372 | Polyclonal | 1:500 |
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| Phospho-p44/42 MAPK (Erk1/2) | (Thr202/Tyr204) | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell Signaling, 9101 | Polyclonal | 1:1000 |
| MAPK (Erk1/2) | / | Anti-MAPK (Erk1/2) | Millipore, 06-182 | Polyclonal | 1:1000 |
| Phospho-eIF2α | (Ser51) | Phospho-eIF2α (Ser51) (D9G8) rabbit mAb | Cell Signaling, 3398 | Monoclonal | 1:1000 |
| eIF2α | / | eIF2α (D7D3) rabbit mAb | Cell Signaling, 5324 | Monoclonal | 1:1000 |
| NF-κB | / | NF-κB p65 (C-20) | Santa Cruz Biotechnology, Inc, sc-372 | Polyclonal | 1:500 |
Antibody Table
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| Phospho-p44/42 MAPK (Erk1/2) | (Thr202/Tyr204) | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell Signaling, 9101 | Polyclonal | 1:1000 |
| MAPK (Erk1/2) | / | Anti-MAPK (Erk1/2) | Millipore, 06-182 | Polyclonal | 1:1000 |
| Phospho-eIF2α | (Ser51) | Phospho-eIF2α (Ser51) (D9G8) rabbit mAb | Cell Signaling, 3398 | Monoclonal | 1:1000 |
| eIF2α | / | eIF2α (D7D3) rabbit mAb | Cell Signaling, 5324 | Monoclonal | 1:1000 |
| NF-κB | / | NF-κB p65 (C-20) | Santa Cruz Biotechnology, Inc, sc-372 | Polyclonal | 1:500 |
| Peptide/Protein Target . | Antigen Sequence (If Known) . | Name of Antibody . | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody . | Species Raised in; Monoclonal or Polyclonal . | Dilution Used . |
|---|---|---|---|---|---|
| Phospho-p44/42 MAPK (Erk1/2) | (Thr202/Tyr204) | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell Signaling, 9101 | Polyclonal | 1:1000 |
| MAPK (Erk1/2) | / | Anti-MAPK (Erk1/2) | Millipore, 06-182 | Polyclonal | 1:1000 |
| Phospho-eIF2α | (Ser51) | Phospho-eIF2α (Ser51) (D9G8) rabbit mAb | Cell Signaling, 3398 | Monoclonal | 1:1000 |
| eIF2α | / | eIF2α (D7D3) rabbit mAb | Cell Signaling, 5324 | Monoclonal | 1:1000 |
| NF-κB | / | NF-κB p65 (C-20) | Santa Cruz Biotechnology, Inc, sc-372 | Polyclonal | 1:500 |
Our results were confirmed in SaOS-2 and MSC extract samples, using an ELISA kit for the p65 subunit of NF-κB following the manufacturer’s instructions (Fisher Scientific).
Quantification of mRNA expression by qPCR
Total RNA was isolated from MSC, Ob cells and SaOS-2 cells seeded for 3 days before treatment in 6-well plates at a density of 10.4 × 103 cells/cm2, using AURUM kit (Bio-Rad) according to the manufacturer’s protocol. Total RNA was reverse transcribed using the iScriptcDNA synthesis kit (Bio-Rad).
qPCRs were performed using a CFX96 thermal system (Bio-Rad) in a total reaction volume of 20 μL containing 3mM MgCl2, 0.3μM (each) forward and reverse primers (Eurogentec), 10-μL SYBR Green mix (Bio-Rad), and 2-μL cDNA (10 ng/μL). The cycling program was as follows: 95°C for 3 minutes followed by 40 cycles at 95°C for 30 seconds and 60°C for 30 seconds. All reactions were performed in duplicate. Expression of Toll-like receptor 4 (TLR4) was quantified by qPCR using the standard curve method (38) and the appropriate primer sets (Supplemental Table 1). Relative mRNA expression of the other target genes was evaluated using the comparative threshold cycle method (2−ΔΔCt) and adequate primer sets (Supplemental Table 2). Hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as housekeeping gene.
To study X-box binding protein 1 (XBP1) splicing, cDNAs were amplified by standard PCR (cycling program: 94°C for 45 s, 58°C for 20 s, and 72°C for 1 min; 24 cycles) using XBP1-specific primers (Supplemental Table 1). XBP1 expression was normalized for HPRT1 expression. A total of 10 μL of the PCR mixture was analyzed on a 2% agarose gel, and bands were visualized using Fusion UV System equipment (Vilber Lourmat).
Western blotting
Human MSC treated with FA were lysed with Laemmli buffer (10% glycerol, 0.5mM dithiothreitol, 63mM Tris-HCl, and 1% sodium dodecyl sulfate (SDS); pH 6.8) containing a cocktail of proteases and phosphatases inhibitors (Roche Diagnostics). Proteins were quantified by a paper dye binding assay. Equal protein amounts were separated by SDS-PAGE using 10% or 12% polyacrylamide gels. Proteins were transferred on polyvinylidene difluoride membranes Immobilion-P (Millipore) and immunolabeled overnight at 4°C using the next antibodies: antiphospho-eukaryotic initiation factor 2 (eIF2)αSer51 and antiphospho-ERKThr202/Tyr204 (Table 1). The secondary antibodies peroxidase-conjugated ECL (GE healthcare) were used for 1 hour at room temperature. The immune complexes were detected by using the enhanced chemiluminescence method (Western Lightning Plus-ECL; PerkinElmer, Inc). Band densities were quantified by ImageJ program (National Institutes of Health). Results were expressed as relative ratio between the phospho- and the total proteins, which were detected with the next antibodies: anti-eIF2α or anti-MAPK (Table 1).
Measurement of IL-6 and IL-8 production
Supernatants of MSC were collected after 48 hours of treatment with FA, aliquoted, and frozen at −20°C until use. IL-6 and IL-8 concentrations were measured by ELISA according to the manufacturer’s protocol (Duoset; R&D Systems).
Detection of intracellular lipid droplets (LD) and characterization of their neutral lipids content
After 16 hours of incubation with FA, SaOS-2 cells were washed twice with PBS and fixed with 4% paraformaldehyde for 15 minutes. Neutral lipids accumulated in intracellular LD were stained with Oil Red O (Sigma-Aldrich) for 2 hours and rinsed twice with PBS before microscopic observation. To evaluate the cellular content of neutral lipids, cells were washed with PBS, scrapped and collected in 1-mL lysis buffer (1% SDS, 60mM Tris-HCl, and 10mM EDTA). Total lipids were extracted with chloroform/methanol/water (2:2:1). Isolation of cellular neutral lipids and analysis of their FA content were performed according to Louis et al (39).
Statistical analysis
Data are presented as mean ± SEM. Values were determined from at least 3 independent experiments. Statistical analysis was performed using Student’s t test or one-way ANOVA followed by t test with the Bonferroni correction for multiple comparisons. Differences between groups were considered as statistically significant at P < .05.
Results
Ole prevents Palm-induced lipotoxicity
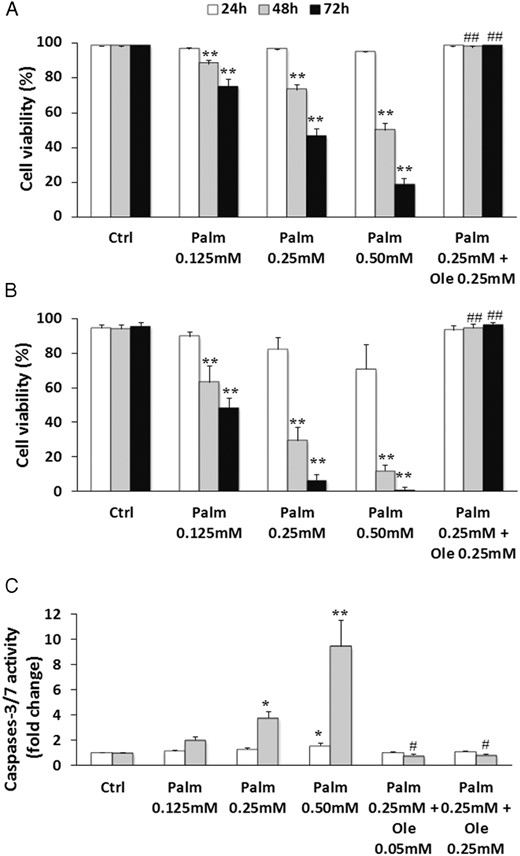
To evaluate the sensitivity of human MSC and Ob to lipotoxicity, cells were exposed to increasing concentrations of Palm (0.125mM–0.50mM) and/or Ole (0.25mM) for 24–72 hours. Palm elicited cell mortality in a time- and dose-dependent manner in both cell types, and our data demonstrated that Ob were more sensitive to lipotoxicity than MSC. Indeed, as showed in Figure 1, A and B, a treatment for 48 hours with 0.50mM of the saturated FA reduced cell viability to 50 ± 3.9% in MSC (P < .001 vs Ctrl) and to 11.8 ± 3.4% in Ob (P < .001 vs Ctrl). Similar observations were obtained in SaOS-2 cells (Supplemental Figure 2A). By contrast, Ole did not affect cell viability (data not shown) but, rather, completely prevented the harmful effect of Palm (Figure 1, A and B). The cytoprotective action of Ole was maintained even when the culture period was extended to 7 days and when the concentration of Ole was decreased to 0.05mM (data not shown).
Ole suppressed Palm-induced cytotoxicity in MSC and Ob. MSC (A and C) and Ob (B) were treated with increasing concentrations of Palm (0.125mM–0.50mM) ± Ole (0.05mM or 0.25mM) for the indicated times. A and B, Cell viability was quantified by nuclear staining with Hoechst and propidium iodide. Values are mean ± SEM of 15 (MSC) or 5 (Ob) individual experiments. **, P < .001 vs appropriate control condition (Ctrl; 24, 48, or 72 hours); ##, P < .001 vs 0.25mM Palm. C, Caspases-3/7 activity was measured using the Caspases-3/7 Glo assay. Values are expressed relative to Ctrl and are mean ± SEM of 7 individual experiments. *, P < .01; **, P < .001 vs appropriate control (Ctrl, 24 or 48 hours); #, P < .01 vs 0.25mM Palm.
To determine whether cell death was associated with apoptosis, caspases-3/7 activity was evaluated after 24 and 48 hours of treatment. Palm toxicity coincided with a dose- and time-dependent increase of caspases-3/7 activity in MSC (Figure 1C). The beneficial effect of Ole on Palm cytotoxicity was reflected by a complete suppression of caspases activation, which persisted even when Ole was tested at 0.05mM (Figure 1C). Similar results were obtained in SaOS-2 cells (Supplemental Figure 2B) and Ob (Supplemental Figure 2C).
Ole abolishes Palm-induced ERK and NF-κB activation
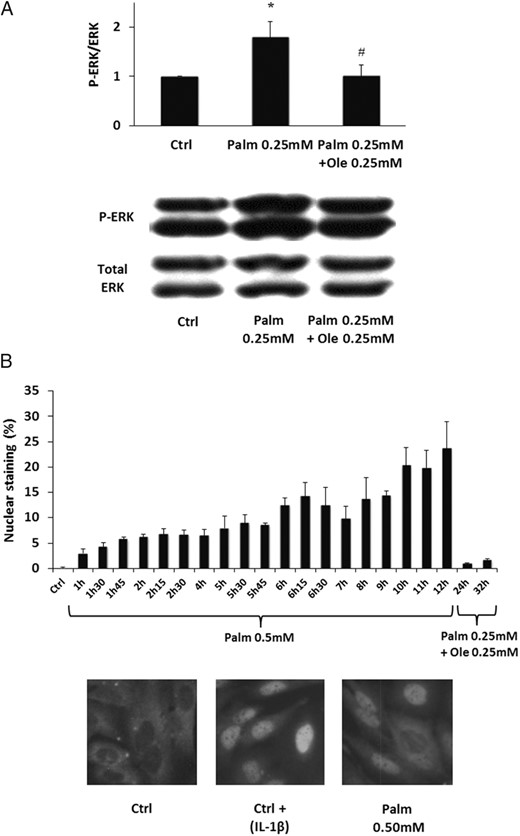
ERK and NF-κB pathways control cell survival. We therefore evaluated the effects of Palm and Ole on ERK activation. Our results showed that Palm stimulated ERK phosphorylation in MSC, which was suppressed by addition of Ole (Figure 2A). Similar results were obtained with SaOS-2 cells (Supplemental Figure 3).
Ole blocked Palm-induced activation of ERK and NF-κB. A, MSC were treated for 48 hours with 0.25mM Palm ± 0.25mM Ole. ERK phosphorylation was evaluated by Western blotting and normalized for total ERK. The Western blotting is representative of 1 out of 4 individual experiments. Values are mean ± SEM. *, P < .05 vs Ctrl; #, P < .05 vs 0.25mM Palm. B, SaOS-2 cells were treated with 0.50mM Palm or 0.25mM Palm plus 0.25mM Ole. Nuclear translocation of NF-κB was regularly evaluated by immunocytochemistry. Values presented as the percentage of stained nuclei are mean ± SEM of 6 individual experiments. Representative immunocytochemistry results of NF-κB nuclear translocation. IL-1β was used as positive control.
In parallel, we evaluated the effect of Palm on activation of NF-κB, a transcription factor ensuring a proapoptotic role in Ob (40). As illustrated in Figure 2B, Palm stimulated NF-κB nuclear translocation in SaOS-2 cells, which did not occur when Palm was associated with Ole. Similar results were obtained in MSC by ELISA (Supplemental Figure 4).
Ole blocks Palm-induced proinflammatory response
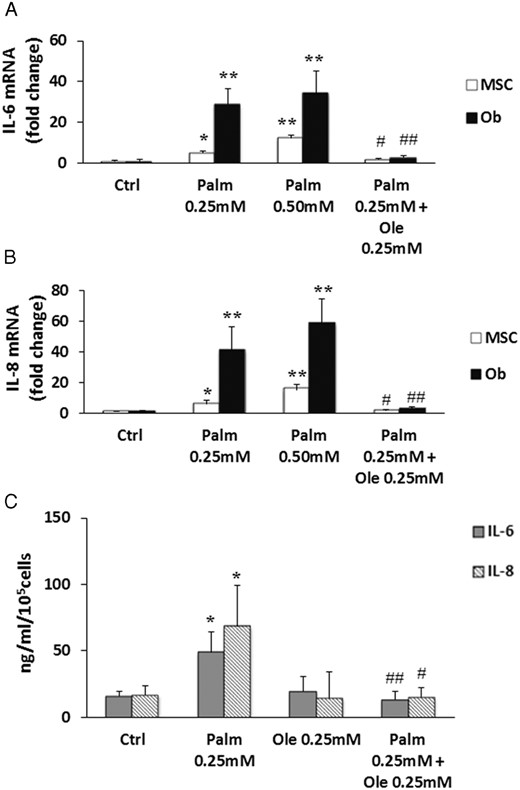
In immune cells, the activation of NF-κB and ERK pathways induces an inflammation process through the transcriptional regulation of cytokines and chemokines. MSC are known to secrete soluble factors in response to stress conditions such as hypoxia or hyperglycemia (41, 42). However, little information is available concerning the effects of Palm and Ole on cytokines regulation in MSC and Ob. Our results showed that, after 24 hours of treatment, Palm triggered IL-6 (Figure 3A) and IL-8 (Figure 3B) expression in MSC and Ob, whereas it did not affect chemokine (C-C motif) ligand 2 (CCL2, also called MCP1) expression (data not shown). Of note, Palm-induced cytokines expression was higher in Ob than in MSC (Figure 3, A and B). Coincubation of the saturated FA with Ole prevented IL-6 and IL-8 gene up-regulation in both cell types (Figure 3, A and B). In parallel, we assessed IL-6 and IL-8 secretion in MSC. Palm triggered the secretion of both cytokines, which was abrogated by Ole (Figure 3C), as observed for gene expression.
Ole prevented IL-6 and IL-8 expression stimulated by Palm. MSC and Ob were treated for 24 hours with increasing concentrations of Palm (0.25mM–0.50mM) ± 0.25mM Ole. IL-6 (A) and IL-8 (B) expressions were quantified by qPCR using the ΔΔCT method compared with Ctrl. Values were normalized for HPRT1 expression and are represented as mean ± SEM of 6 individual experiments. *, P < .01 and **, P < .001 vs Ctrl; #, P < .01 and ##, P < .001 vs 0.25mM Palm. C, IL-6 and IL-8 secretions were quantified in supernatants of MSC treated 48 hours with 0.25mM Palm ± 0.25mM Ole using ELISA kit. Values were normalized for 100 000 cells and are represented as mean ± SEM of 7 individual experiments. *, P < .05 vs Ctrl; #, P < .05 and ##, P < .01 vs 0.25mM Palm.
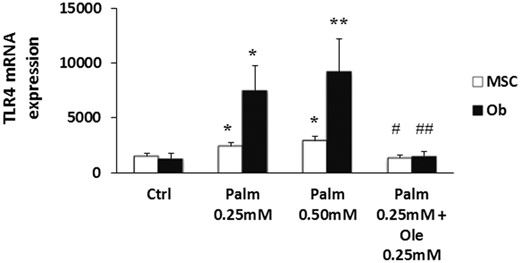
TLRs detect exogenous pathogen-associated molecular patterns and initiate cytokines and chemokines secretion via ERK and NF-κB pathways activation. Interestingly, TLR4 is also able to trigger immune responses after the detection of endogenous ligands such as the long-chain FA Palm and stearate (43). We observed that, after 24 hours of treatment, Palm increased dose dependently TLR4 expression in MSC and, to a more marked extent, in Ob (Figure 4). Ole alone did not alter TLR4 expression (data not shown) but repressed Palm-induced gene up-regulation (Figure 4). Similar data were obtained in SaOS-2 cells (data not shown). Therefore, to examine the contribution of TLR4 in Palm-induced lipotoxicity, we used MSC isolated from the Warthon jelly (WJ-MSC), because they do not express TLR4 (44). We observed that WJ-MSC were as susceptible to lipotoxicity as MSC isolated from the bone marrow (Supplemental Figure 5).
Ole abolished Palm-induced expression of TLR4. MSC and Ob were treated for 24 hours with Palm (0.25mM–0.50mM) ± 0.25mM Ole. TLR4 expression was quantified by qPCR using the standard curve method and normalized for HPRT1.Values are mean ± SEM of 6 individual experiments. *, P < .05 and **, P < .01 vs Ctrl; #, P < .05 and ##, P < .01 vs Palm 0.25mM.
Ole abrogates Palm-induced endoplasmic reticulum (ER) stress activation
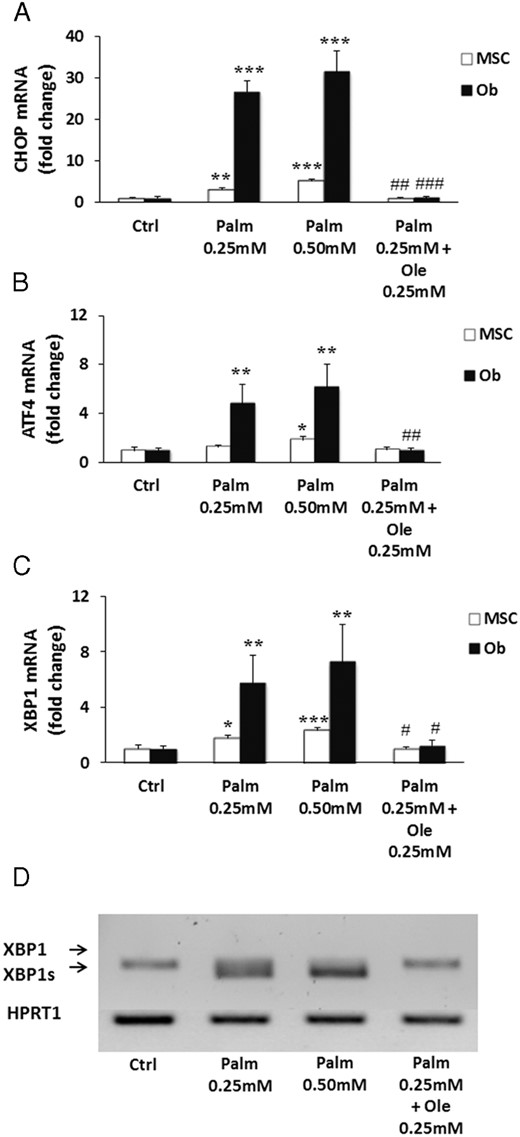
ER stress was recently implicated in Palm-induced apoptosis in human MSC (45). Therefore, we evaluated whether the protective action of Ole could be mediated through prevention of ER stress initiation in MSC and Ob by different approaches. First, we measured the expression levels of well-known ER stress marker genes such as the transcription factors C/emopamil binding protein (C/EBP) homologous protein, activating transcription factor 4, and XBP1. A 24-hour treatment with Palm up-regulated the expression of the selected genes in both MSC and Ob (Figure 5, A–C). Interestingly, the impact of the saturated FA on gene expression was more marked in Ob than in MSC. Moreover, Palm activated the cleavage of XBP1 mRNA (Figure 5D). Ole abolished Palm-induced gene up-regulation and XBP1 splicing in both cell types (Figure 5).
Ole blocked ER stress markers expression and activation of Palm-induced XBP1 splicing. MSC and Ob were treated for 24 hours with 0.25mM Palm ± 0.25mM Ole. C/EBP homologous protein (CHOP) (A), activating transcription factor 4 (ATF4) (B), and XBP1 (C) expression were quantified by qPCR using the ΔΔCT method. Values were normalized for HPRT1 expression and are expressed as the ΔΔCT compared with Ctrl. Results are mean ± SEM of 6 individual experiments. *, P < .05; **, P < .01; ***, P < .001 vs Ctrl; #, P < .05; ##, P < .01; ###, P < .001 vs Palm 0.25mM. D, Representative PCR results for XBP1 splicing, compared with HPRT1 expression, of 1 out of 6 individual experiments in MSC.
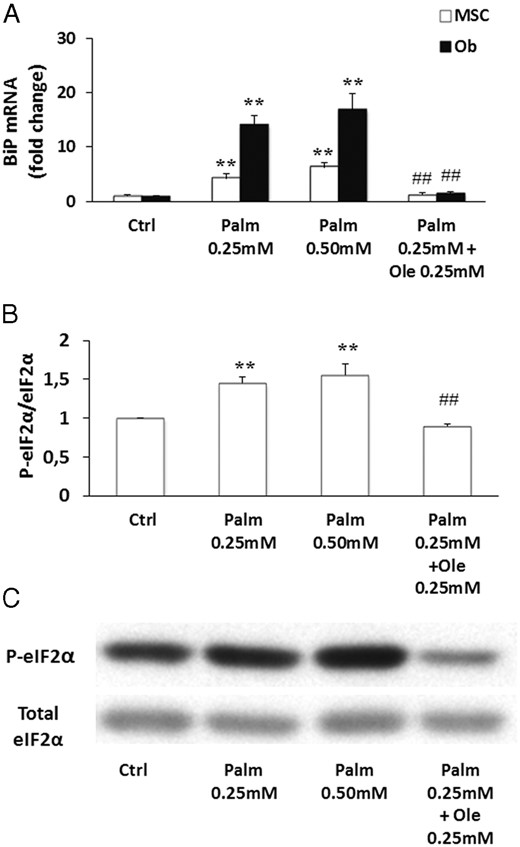
Secondly, we evaluated the activation of the unfolded protein response (UPR), a signaling network initiated in response to severe ER stress. The shutdown of protein synthesis was assessed by measuring the phosphorylation of the α-subunit of the eIF2 and the expression of the Ca2+-sensitive chaperone binding immunoglobulin protein (BiP) was determined by qPCR. Our results demonstrated that Palm treatment led to overexpression of BiP (Figure 6A) and increased eIF2α phosphorylation in MSC (Figure 6, B and C). Addition of Ole suppressed BiP expression (Figure 6A) and restored eIF2α phosphorylation to its control level (Figure 6, B and C). Similar results were obtained in SaOS-2 cells (data not shown).
Ole prevented Palm-triggered eIF2α activation and BiP expression. MSC were treated for 24 and 48 hours with increasing concentrations of Palm (0.25mM–0.50mM) ± Ole 0.25mM. A, BiP expression was quantified by qPCR using the ΔΔCT. Values were normalized for HPRT1 expression and are expressed as the ΔΔCT compared with the control (Ctrl). Results are mean ± SEM of 6 individual experiments. B, Expression of phosphorylated eIF2α was evaluated by Western blotting and normalized for total eIF2α. Values are mean ± SEM. **, P < .001 vs Ctrl; ##, P < .001 vs Palm 0.25mM. C, The Western blotting figure is representative of 1 out of 4 individual experiments.
Ole, but not Palm, induces LDs formation
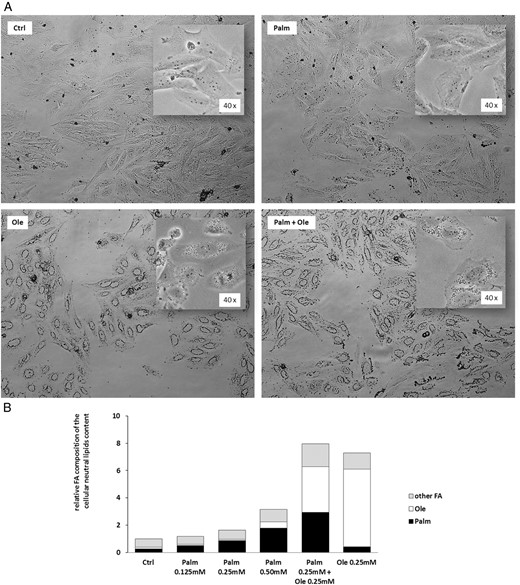
In situations of excessive FA exposure, TGs may accumulate in intracellular LD that have now gain recognition as dynamic organelles involved in various cellular processes (46). We therefore evaluated the capacity of Palm and Ole to induce LD formation in Ob. As illustrated in Figure 7A, SaOS-2 cells cultured for 16 hours in control conditions or in the presence of Palm did not present LD. However, Ole, tested alone or in the concomitant presence of Palm, triggered LD formation (Figure 7A). We further quantified and characterized the neutral lipids content (mainly composed of mono-, di-, and TG) of SaOS-2 cells exposed to Palm and/or Ole (Figure 7B). In the absence of FA, the cellular neutral lipids content amounted to 3.4 ± 1.4 μg/106 cells. Increased concentrations of Palm (0.125mM–0.50mM) tended to augment (P = .08) the cellular neutral lipids content whereas 0.25mM Ole was sufficient to induce a 7.3-fold increase (P = .018) of total neutral lipids (Figure 7B). When compared with 0.25mM Ole alone, the concomitant presence of Palm and Ole (0.25mM each) did not modify the total lipid content. Nevertheless, when compared with 0.25mM Palm, the addition of Ole increased by 3.4-fold the incorporation of Palm into the neutral lipids fraction (P < .001) (Figure 7B).
Ole, but not Palm, enhanced LD formation and promoted Palm incorporation into the neutral lipids fraction. SaOS-2 cells were treated for 16 hours with increasing concentrations of Palm (0.125mM–0.50mM) ± 0.25mM Ole. A, LD were identified by Oil Red staining. B, Total cellular neutral lipids content and FA composition were evaluated by gas chromatography. Values are expressed relative to Ctrl and are means of 3 individual experiments.
Discussion
Fat is an integral component of the bone marrow microenvironment, but its physiological function and its role in the pathogenesis of metabolic bone diseases are still under debate. In osteoporotic patients and in the context of ageing, an inverse relation between marrow adipose tissue (MAT) and bone mass is now well established (47, 48). However, in animal models, obesity or a high-fat diet may trigger various bone responses ranging from preserved bone density (49) to decreased bone mass and quality (50–52). In humans, obesity was previously often correlated with a higher bone mass density (53, 54), but it may also adversely alter bone quality and contribute to an increased fracture risk (55, 56). Recent studies demonstrate that bone mass and MAT are inversely correlated in healthy subjects (57, 58). On the whole, these data demonstrate the complexity of the relationship between exogenous lipid intake, MAT, and bone health. Of note, the concept that the FA profile as well as the amount of marrow adipocytes may be relevant for skeletal preservation is emerging (59, 60).
Recent experimental data suggest that FA and lipotoxicity could play a role in the initiation and/or evolution of metabolic bone diseases such as osteoporosis.
In the present study we evaluated the effect of Palm and Ole, the most common saturated and monounsaturated FA found in human serum and in the Western diet (30). Of note, Palm was recently identified as one of the FA predominantly liberated in the culture medium by MSC-derived adipocytes (25). The effect of Palm and Ole were extensively studied in cell types implicated in disorders of energy metabolism (ie, hepatocytes, insulin-producing pancreatic β-cells, myocytes). However, the concept of lipotoxicity recently emerged in skeletal cells and the few studies conducted on human bone marrow-derived MSC and MSC-derived Ob focused on the action of Palm (25, 31, 35, 36, 45). The studies addressing the effect of Ole are rare and diverging. Although the recent work of Fillmore et al (61) demonstrated that Ole abrogated Palm-induced impairment of MSC metabolism, 2 previous studies indicated that Ole is toxic for MSC, does not prevent Palm-induced toxicity and increases Palm-triggered apoptosis (31, 62).
We demonstrated that Palm and Ole inversely regulate cell death and function of human MSC and Ob. We confirmed that Palm elicits cell death and showed that Ob are more sensitive to lipotoxicity than MSC. We established that the monounsaturated FA Ole is not toxic for MSC and Ob but that it completely abrogates Palm-induced lipotoxicity and proinflammatory response.
Depending on the cell type, lipotoxicity is related to the initiation of different cellular mechanisms (21). We thus further clarified the molecular mechanisms underlying the cytotoxic and cytoprotective actions of the 2 FA in MSC and Ob, because their understanding may pave the way for innovative therapeutic approaches.
ERK activation may lead to cell death or survival. A recent study showed that Palm activates ERK phosphorylation in MSC (45), whereas contradictory data were obtained in Ob (31, 35). We showed that Palm triggers ERK phosphorylation in MSC and Ob; Ole does not affect ERK pathway but prevents its activation by Palm. Furthermore, we demonstrated that Palm activates the transcription factor NF-κB, a phenomenon counteracted by Ole. NF-κB plays a proapoptotic role in Ob and its activation was associated with lipotoxicity-induced ER stress in other cell types (40, 63). Thus, Ole could protect MSC and Ob from Palm-induced lipotoxicity by preventing the initiation of 2 proapoptotic pathways.
In MSC, ERK and NF-κB pathways regulate cytokines and chemokines secretion and can be activated after TLRs stimulation by exogenous ligands. The effects of FA on cytokines production and TLRs expression have not yet been investigated in human MSC and Ob. We showed that Palm triggers TLR4, IL-6, and IL-8 expression and secretion that were completely suppressed by Ole. Palm-induced cytokines and TLR4 expression was higher in Ob than in MSC. Chronic inflammation is associated with aging and induces bone loss and osteoporosis (64). Interestingly, a growing number of studies related to metabolic diseases points to a link between enhanced circulating FA levels and the induction of a chronic inflammatory process via TLRs stimulation (65). TLRs perpetuate inflammatory responses through the recognition of exogenous pathogen-associated molecular patterns and endogenous ligands. For example, TLR4 is able to trigger immune responses after the detection of long-chain FA (43). MSC constitutively express the proinflammatory cytokines IL-6 and IL-8. However, the overproduction of these cytokines stimulates the differentiation of osteoclast precursor cells to mature osteoclasts (66, 67). By enhancing IL-6 and IL-8 production, Palm could thus promote a proinflammatory state favoring bone resorption and impairing bone formation. In line with this hypothesis, IL-6 and IL-8 levels are increased in both the synovial fluid and the serum of patients suffering from chronic inflammatory diseases predisposing to osteoporosis (68, 69).
To clarify the role of TLR4 in Palm-induced lipotoxicity, we used WJ-MSC that do not express TLR4 (44). WJ-MSC were as susceptible to Palm as MSC isolated from bone marrow, suggesting that TLR4 does not mediate lipotoxicity in MSC and Ob. However, TLR2 may also recognize FA (43). TLR2 is expressed in WJ-MSC (44), and we observed that it is up-regulated by Palm (data not shown). Therefore, the functional role of TLR4 and/or TLR2 in Palm-induced lipotoxicity should not be excluded and requires further investigations.
Saturated FA are major ER-stress inducers in pancreatic β-cells (70), and it has recently been shown that FA-induced MSC apoptosis implicate ER stress activation (45). Altogether, our results showed that Palm triggered ER stress and UPR in both MSC and Ob. The ER stress response is more severe in Ob, which correlates with their higher susceptibility to lipotoxicity. Moreover, we established that Ole exerts its protective action by abolishing Palm-induced ER dysfunctions. The ER plays a pivotal role in the regulation of lipid and protein metabolism. The accumulation of misfolded proteins or other stressors in the ER initiates a cellular adaptive program, known as the UPR, to reestablish the cellular equilibrium: although the inhibition of general protein synthesis prevents further overloading of the ER, the simultaneous production of specific chaperones favors the refolding of misfolded proteins. However, prolonged or extreme ER stress overwhelms the normal ER protein folding machinery, triggers activation of proapoptotic genes and leads to cell death (63).
In situations of excessive FA exposure, the relative toxicity of FA is related to their capacity to be directed to distinct metabolic fates. For example, palmitoylation was recently implicated in Palm-induced toxicity in human osteoblasts (36). On the other hand, it is known that Palm is poorly incorporated into TG and causes apoptosis, whereas Ole stimulates TG accumulation and is well tolerated. Monounsaturated FA thus promote the detoxification of saturated FA by fostering their esterification into TG so that the potentially toxic lipids are safely stored within LD (71). LD formation in nonadipose cells in response to lipid overload was therefore proposed as an initial cellular defense against lipotoxicity (72). The role of TG accumulation in cell survival is, however, somewhat controversial. In the insulin producing pancreatic β-cells, it was described as cytotoxic (73) or cytoprotective (37).
We thus evaluated the capacity of Palm and Ole to induce LD formation in Ob. Ole, but not Palm, triggers LD formation and increases the cellular neutral lipids content. Moreover, in the concomitant presence of Palm, Ole favors the incorporation of the saturated FA into the neutral lipids fraction. These observations suggest that Ole promotes FA storage and may therefore serve its protective function by favoring Palm esterification and incorporation into stable LD.
In conclusion, several pathways are involved in Palm-induced toxicity in human MSC and Ob. The lipotoxic action exerted by Palm is prevented by the monounsaturated FA Ole via induction of TG synthesis and inhibition of ERK activation and ER stress initiation. In addition, Palm, but not Ole, has a proinflammatory effect that may compromise bone health.
Interestingly, Drosatos-Tampakaki et al have recently shown that Palm is not toxic for osteoclasts, the bone resorbing cells and enhances osteoclastogenesis, thus favoring bone resorption (74). On the contrary, Ole does not support osteoclast differentiation but inhibits Palm-induced osteoclastogenesis by increasing TG accumulation and inducing LD formation (74).
The present study, combined with the work of Drosatos-Tampakaki et al (74), demonstrates that saturated and monounsaturated FA have opposite actions on osteoblasts and osteoclasts. Therefore, although Palm could impair bone formation and promote bone resorption, Ole could have a protective action on bone cells by counteracting those effects. Further studies should now be pursued in animal models of bone diseases associated with alterations of lipid metabolism, such as osteoporosis, to establish the impact of FA on bone mass and evaluate whether the manipulation of dietary FA composition could affect skeletal health.
Acknowledgments
We thank Jessica Lechanteur for technical support and Dr Laurence Lagneaux for the gift of MSC isolated from Wharton jelly.
This work was supported by the Fonds National de la Recherche Scientifique (Convention J.088.13CDR) and by the Daiichi Sankyo-Belgian Bone Club Young Investigator Award.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- ALP
alkaline phosphatase
- BiP
binding immunoglobulin protein
- CD
clusters of differentiation
- FA
fatty acid
- FBS
fetal bovine serum
- 2−ΔΔCt
comparative threshold cycle method
- eIF2
eukaryotic initiation factor 2
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- HPRT1
hypoxanthine phosphoribosyltransferase 1
- LD
lipid droplet
- MAT
marrow adipose tissue
- MSC
mesenchymal stem cell
- NF-κB
nuclear factor-κB
- Ob
osteoblastic cell
- Ole
oleate
- Palm
palmitate
- qPCR
quantitative PCR
- TG
triglyceride
- TLR4
Toll-like receptor 4
- XBP1
X-box binding protein 1
- UPR
unfolded protein response
- WJ-MSC
MSC isolated from Warthon jelly.
References