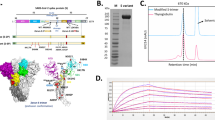
Abstract
The protective efficacies of current licensed vaccines against COVID-19 have significantly reduced as a result of SARS-CoV-2 variants of concern (VOCs) which carried multiple mutations in the Spike (S) protein. Considering that these vaccines were developed based on the S protein of the original SARS-CoV-2 Wuhan strain, we designed a recombinant plasmid DNA vaccine based on highly conserved and immunogenic B and T cell epitopes against SARS-CoV-2 Wuhan strain and the Omicron VOC. Literature mining and bioinformatics were used to identify 6 immunogenic peptides from conserved regions of the SARS-CoV-2 S and membrane (M) proteins. Nucleotide sequences encoding these peptides representing highly conserved B and T cell epitopes were cloned into a pVAX1 vector to form the pVAX1/S2-6EHGFP recombinant DNA plasmid vaccine. The DNA vaccine was intranasally or intramuscularly administered to BALB/c mice and evaluations of humoral and cellular immune responses were performed. The intramuscular administration of pVAX1/S2-6EHGFP was associated with a significantly higher percentage of CD8+ T cells expressing IFN-γ when compared with the empty vector and PBS controls. Intramuscular or intranasal administrations of pVAX1/S2-6EHGFP resulted in robust IgG antibody responses. Sera from mice intramuscularly immunized with pVAX1/S2-6EHGFP were found to elicit neutralizing antibodies capable of SARS-CoV-2 Omicron variant with the ACE2 cell surface receptor. This study demonstrated that the DNA vaccine construct encoding highly conserved immunogenic B and T cell epitopes was capable of eliciting potent humoral and cellular immune responses in mice.







Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of the study are available within the article and its Supplementary section. Raw data that support the findings of the study are available from the corresponding author, upon reasonable request.
References
Sannathimmappa M, Nambiar V. COVID-19: An insight into SARS-CoV-2 pandemic originated at Wuhan City in Hubei province of China. Journal of Infectious Diseases and Epidemiology. 2020;6. https://doi.org/10.23937/2474-3658/1510146.
WHO: WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ (2023). Accessed.
Ahmed S, Khan S, Imran I, Al Mughairbi F, Sheikh FS, Hussain J, et al. Vaccine development against COVID-19: Study from pre-clinical phases to clinical trials and global use. Vaccines. 2021;9(8):836.
Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado-Garcia J, O'Horo JC, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv : the preprint server for health sciences. 2021. https://doi.org/10.1101/2021.08.06.21261707.
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. https://doi.org/10.1016/S0140-6736(20)32661-1.
Zhang Y, Belayachi J, Yang Y, Fu Q, Rodewald L, Li H, et al. Real-world study of the effectiveness of BBIBP-CorV (Sinopharm) COVID-19 vaccine in the Kingdom of Morocco. BMC Pub Health. 2022;22(1):1584. https://doi.org/10.1186/s12889-022-14016-9.
Jungreis I, Sealfon R, Kellis M. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat Commun. 2021;12(1):2642. https://doi.org/10.1038/s41467-021-22905-7.
Alanagreh L, Alzoughool F, Atoum M. The Human Coronavirus Disease COVID-19: Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. Pathogens. 2020;9(5). https://doi.org/10.3390/pathogens9050331.
Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727–34. https://doi.org/10.1073/pnas.2003138117.
Rastogi M, Pandey N, Shukla A, Singh SK. SARS coronavirus 2: from genome to infectome. Respiratory Res. 2020;21(1):318. https://doi.org/10.1186/s12931-020-01581-z.
Cosar B, Karagulleoglu ZY, Unal S, Ince AT, Uncuoglu DB, Tuncer G, et al. SARS-CoV-2 Mutations and their Viral Variants. Cytokine Growth Factor Rev. 2022;63:10–22. https://doi.org/10.1016/j.cytogfr.2021.06.001.
Farhud DD, Mojahed N. SARS-COV-2 Notable Mutations and Variants: A Review Article. Iran J Public Health. 2022;51(7):1494–501. https://doi.org/10.18502/ijph.v51i7.10083.
Magazine N, Zhang T, Wu Y, McGee MC, Veggiani G, Huang W. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses. 2022;14(3):640.
Agerer B, Koblischke M, Gudipati V, Montaño-Gutierrez LF, Smyth M, Popa A, et al. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8(+) T cell responses. Sci Immunol. 2021;6(57). https://doi.org/10.1126/sciimmunol.abg6461.
Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–6. https://doi.org/10.1038/s41586-021-04387-1.
Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nature Medicine. 2022;28(3):486-9. https://doi.org/10.1038/s41591-022-01704-7.
Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, et al. Sensitivity of SARS-CoV-2 B117 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857):136-41. https://doi.org/10.1038/s41586-021-03412-7.
Hachmann NP, Miller J, Collier A-rY, Ventura JD, Yu J, Rowe M, et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. New England Journal of Medicine. 2022;387(1):86-8. https://doi.org/10.1056/NEJMc2206576.
Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. New England Journal of Medicine. 2021;384(20):1885-98. https://doi.org/10.1056/NEJMoa2102214.
Noh JY, Jeong HW, Shin E-C. SARS-CoV-2 mutations, vaccines, and immunity: implication of variants of concern. Signal Transduct Target Therapy. 2021;6(1):203.
Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021. https://doi.org/10.1101/2021.01.25.427948.
Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, Peacock SJ, et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21(3):162–77. https://doi.org/10.1038/s41579-022-00841-7.
Chakraborty C, Bhattacharya M, Sharma AR, Mallik B. Omicron (B.1.1.529) - A new heavily mutated variant: Mapped location and probable properties of its mutations with an emphasis on S-glycoprotein. International Journal of Biological Macromolecules. 2022;219:980-97. https://doi.org/10.1016/j.ijbiomac.2022.07.254.
Willett BJ, Grove J, MacLean OA, Wilkie C, De Lorenzo G, Furnon W, et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nature Microbio. 2022;7(8):1161–79. https://doi.org/10.1038/s41564-022-01143-7.
Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mole Therapy. 2019;27(4):757–72. https://doi.org/10.1016/j.ymthe.2019.01.020.
Moyle PM, Toth I. Modern Subunit Vaccines: Development, Components, and Research Opportunities. ChemMedChem. 2013;8(3):360–76. https://doi.org/10.1002/cmdc.201200487.
Smith TR, Patel A, Ramos S, Elwood D, Zhu X, Yan J, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nature Commun. 2020;11(1):2601.
Chalkias S, Whatley JL, Eder F, Essink B, Khetan S, Bradley P, et al. Original SARS-CoV-2 monovalent and Omicron BA.4/BA.5 bivalent COVID-19 mRNA vaccines: phase 2/3 trial interim results. Nat Med. 2023;29(9):2325-33. https://doi.org/10.1038/s41591-023-02517-y.
Mammen P. Mammen J, Tebas P, Agnes J, Giffear M, Kraynyak KA, Blackwood E, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv. 2021:2021.05.07.21256652. https://doi.org/10.1101/2021.05.07.21256652.
Khobragade A, Bhate S, Ramaiah V, Deshpande S, Giri K, Phophle H, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399(10332):1313–21. https://doi.org/10.1016/S0140-6736(22)00151-9.
Tebas P, Kraynyak KA, Patel A, Maslow JN, Morrow MP, Sylvester AJ, et al. Intradermal SynCon® Ebola GP DNA Vaccine Is Temperature Stable and Safely Demonstrates Cellular and Humoral Immunogenicity Advantages in Healthy Volunteers. J Infect Dis. 2019;220(3):400–10. https://doi.org/10.1093/infdis/jiz132.
Huang L, Rong Y, Pan Q, Yi K, Tang X, Zhang Q, et al. SARS-CoV-2 vaccine research and development: Conventional vaccines and biomimetic nanotechnology strategies. Asian J Pharmac Sci. 2021;16(2):136–46. https://doi.org/10.1016/j.ajps.2020.08.001.
Chai KM, Tzeng TT, Shen KY, Liao HC, Lin JJ, Chen MY, et al. DNA vaccination induced protective immunity against SARS CoV-2 infection in hamsterss. PLoS Negl Trop Dis. 2021;15(5):e0009374. https://doi.org/10.1371/journal.pntd.0009374.
Hobernik D, Bros M. DNA Vaccines—How Far From Clinical Use? Int J Molecular Sci. 2018;19(11):3605.
Ingolotti M, Kawalekar O, Shedlock DJ, Muthumani K, Weiner DB. DNA vaccines for targeting bacterial infections. Expert Rev Vaccines. 2010;9(7):747–63. https://doi.org/10.1586/erv.10.57.
Prompetchara E, Ketloy C, Tharakhet K, Kaewpang P, Buranapraditkun S, Techawiwattanaboon T, et al. DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice. PLoS One. 2021;16(3):e0248007. https://doi.org/10.1371/journal.pone.0248007.
Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31:100689. https://doi.org/10.1016/j.eclinm.2020.100689.
Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806–11. https://doi.org/10.1126/science.abc6284.
Alluhaybi KA, Alharbi RH, Alhabbab RY, Aljehani ND, Alamri SS, Basabrain M, et al. Cellular and humoral immunogenicity of a candidate dna vaccine expressing SARS-CoV-2 spike subunit 1. Vaccines. 2021;9(8):852.
Yadav PD, Kumar S, Agarwal K, Jain M, Patil DR, Maithal K, et al. Needle-free injection system delivery of ZyCoV-D DNA vaccine demonstrated improved immunogenicity and protective efficacy in rhesus macaques against SARS-CoV-2. J Med Virol. 2023;95(2):e28484. https://doi.org/10.1002/jmv.28484.
Martins M, do Nascimento GM, Conforti A, Noll JCG, Impellizeri JA, Sanchez E, et al. A linear SARS-CoV-2 DNA vaccine candidate reduces virus shedding in ferrets. Arch Virol. 2023;168(4):124. https://doi.org/10.1007/s00705-023-05746-1.
Dhama K, Dhawan M. COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges. 2022;18(5):2045853. https://doi.org/10.1080/21645515.2022.2045853.
Schultz MD, Suschak JJ. A single intranasal administration of AdCOVID protects against SARS-CoV-2 infection in the upper and lower respiratory tracts. 2022;18(6):2127292. https://doi.org/10.1080/21645515.2022.2127292.
Diallo BK, Ní Chasaide C, Wong TY, Schmitt P, Lee KS, Weaver K, et al. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. npj Vaccines. 2023;8(1):68. https://doi.org/10.1038/s41541-023-00665-3.
Hassan AO, Shrihari S, Gorman MJ, Ying B, Yuan D, Raju S, et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell reports. 2021;36(4):109452. https://doi.org/10.1016/j.celrep.2021.109452.
Ramvikas M, Arumugam M, Chakrabarti SR, Jaganathan KS. Nasal Vaccine Delivery. Micro and Nanotechnology in Vaccine Development. 2017:279-301. https://doi.org/10.1016/B978-0-323-39981-4.00015-4.
Tan CW, Chia WN, Qin X, Liu P, Chen MIC, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nature Biotechnol. 2020;38(9):1073–8. https://doi.org/10.1038/s41587-020-0631-z.
Chang D, Chang X, He Y, Tan KJK. The determinants of COVID-19 morbidity and mortality across countries. Sci Rep. 2022;12(1):5888. https://doi.org/10.1038/s41598-022-09783-9.
Ao D, He X, Hong W, Wei X. The rapid rise of SARS-CoV-2 Omicron subvariants with immune evasion properties: XBB.1.5 and BQ.1.1 subvariants. MedComm (2020). 2023;4(2):e239. https://doi.org/10.1002/mco2.239.
Feng K, Zheng X, Wang R, Gao N, Fan D, Sheng Z, et al. Long-Term Protection Elicited by a DNA Vaccine Candidate Expressing the prM-E Antigen of Dengue Virus Serotype 3 in Mice. Front Cell Infect Microbiol. 2020;10:87. https://doi.org/10.3389/fcimb.2020.00087.
Lim HX, Masomian M, Khalid K, Kumar AU, MacAry PA, Poh CL. Identification of B-Cell Epitopes for Eliciting Neutralizing Antibodies against the SARS-CoV-2 Spike Protein through Bioinformatics and Monoclonal Antibody Targeting. Int Mole Sci. 2022;23(8):4341.
Lim HX, Lim J, Jazayeri SD, Poppema S, Poh CL. Development of multi-epitope peptide-based vaccines against SARS-CoV-2. Biomed J. 2021;44(1):18–30. https://doi.org/10.1016/j.bj.2020.09.005.
Yarmarkovich M, Warrington JM, Farrel A, Maris JM. Identification of SARS-CoV-2 Vaccine Epitopes Predicted to Induce Long-Term Population-Scale Immunity. Cell Reports Medicine. 2020;1(3):100036. https://doi.org/10.1016/j.xcrm.2020.100036.
Deng W, Sweeney RW. Intramuscular injection of a mixture of COVID-19 peptide vaccine and tetanus vaccine in horse induced neutralizing antibodies against authentic virus of SARS-CoV-2 Delta variant. Vaccine: X. 2022;12:100230. https://doi.org/10.1016/j.jvacx.2022.100230.
Heffron AS, McIlwain SJ, Amjadi MF, Baker DA, Khullar S, Sethi AK, et al. The landscape of antibody binding in SARS-CoV-2 infection. bioRxiv. 2021. https://doi.org/10.1101/2020.10.10.334292.
Dërmaku-Sopjani M, Sopjani M. Interactions between ACE2 and SARS-CoV-2 S Protein: Peptide Inhibitors for Potential Drug Developments Against COVID-19. Curr Protein Pept Sci. 2021;22(10):729–44. https://doi.org/10.2174/1389203722666210916141924.
Bertoglio F, Fühner V, Ruschig M, Heine PA, Abassi L, Klünemann T, et al. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021;36(4):109433. https://doi.org/10.1016/j.celrep.2021.109433.
Zhou X, Ye Q. Cellular Immune Response to COVID-19 and Potential Immune Modulators. Frontiers in Immunology. 2021;12. https://doi.org/10.3389/fimmu.2021.646333.
Lee LYY, Izzard L, Hurt AC. A Review of DNA Vaccines Against Influenza. Front Immunol. 2018;9:1568. https://doi.org/10.3389/fimmu.2018.01568.
Silveira MM, Moreira G, Mendonça M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021;267:118919. https://doi.org/10.1016/j.lfs.2020.118919.
Antonis AF, Schrijver RS, Daus F, Steverink PJ, Stockhofe N, Hensen EJ, et al. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J Virol. 2003;77(22):12067–73. https://doi.org/10.1128/jvi.77.22.12067-12073.2003.
Muthumani K, Falzarano D, Reuschel EL, Tingey C, Flingai S, Villarreal DO, et al. A synthetic consensus anti–spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Science translational medicine. 2015;7(301):301ra132-301ra132.
Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–4. https://doi.org/10.1038/nature02463.
Smith TR, Schultheis K, Morrow MP, Kraynyak KA, McCoy JR, Yim KC, et al. Development of an intradermal DNA vaccine delivery strategy to achieve single-dose immunity against respiratory syncytial virus. Vaccine. 2017;35(21):2840–7.
Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586(7830):572–7. https://doi.org/10.1038/s41586-020-2599-8.
Persson G, Restori KH, Emdrup JH, Schussek S, Klausen MS, Nicol MJ, et al. DNA immunization with in silico predicted T-cell epitopes protects against lethal SARS-CoV-2 infection in K18-hACE2 mice. Frontiers in immunology. 2023;14. https://doi.org/10.3389/fimmu.2023.1166546.
Funding
This research was funded by Sunway University Research Grants 2021 (GRTIN-RF-01-2021) and Sunway University Internal Grant Scheme 2023 (GRTIN-IGS(02)-CVVR-11-2023) to Chit Laa Poh and Hui Xuan Lim from the Centre for Virus and Vaccine Research (CVVR), School of Medical and Life Sciences, Sunway University. This study was also supported by Sunway University Internal Grant Scheme 2022 (GRTIN-IGS(02)-DBS-10-2022) to Ayaz Anwar from Department of Biological Science, School of Medical and Life Sciences, Sunway University.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.K., H.X.L., and C.L.P.; data analysis, K.K. and H.X.L.; investigation, K.K., H.X.L., T.S.H.; writing original draft, K.K.; review and editing, K.K., H.X.L., A.A., H.J.S., O.S.K., and C.L.P.; supervision C.L.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalid, K., Lim, H.X., Anwar, A. et al. Preclinical Development of a Novel Epitope-based DNA Vaccine Candidate against SARS-CoV-2 and Evaluation of Immunogenicity in BALB/c Mice. AAPS PharmSciTech 25, 60 (2024). https://doi.org/10.1208/s12249-024-02778-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02778-x