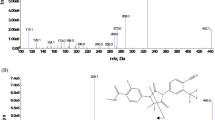
Abstract
Margetuximab was approved for the treatment of advanced HER2+ breast cancer. A feasible analytical technique that can measure this drug was obligatory. In light of this, a novel and thoroughly validated liquid chromatographic (LC)-tandem mass spectrometric (MS/MS) approach was developed for the quantification of margetuximab in rat plasma. The liquid-liquid extraction method was used to extract the analyte from rat plasma. The analyte was separated using acetonitrile and formic acid buffer (30:70) as a mobile phase on Waters, alliance e-2695 model HPLC having Symmetry C18 column, 150 mm × 4.6 mm, 3.5-µm column. The overall runtime was 6 min at a flow rate of 1.0 ml/min. The method showed significant sensitivity and acceptable linearity over the concentration range of 6–120 ng/ml. Accuracy was within 98.51–99.92%. The intraday precision ranged between 0.41 and 8.98% CV. Also, the findings of pharmacokinetic parameters such as Cmax, tmax, AUC0–∞, AUC0–t, and half-life results of margetuximab showed that the technique was helpful for accurately measuring drug concentrations in rat plasma. The method that was developed was useful and effective for quantifying margetuximab.
Graphical abstract











Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Heravi Karimovi M, Pourdehqan M, Jadid Milani M, Foroutan SK, Aieen F. Study of the effects of group counselling on quality of sexual life of patients with breast cancer under chemotherapy at Imam Khomeini Hospital. J Maz Univ Med Sci. 2006;16:43–51.
Sariego J. Breast cancer in the young patient. Am Surg. 2010;76:1397–400.
Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82.
Stoddard FR, Brooks AD, Eskin BA, Johannes GJ. Iodine alters gene expression in the MCF7 breast cancer cell line: evidence for an anti-estrogen effect of iodine. Int J Med Sci. 2008;5:189–96.
Labrecque LG, Barnes DM, Fentiman IS, Griffin BE. Epstein-Barr virus in epithelial cell tumors: a breast cancer study. Cancer Res. 1995;55:39–45.
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30.
Walsh T, Casadei S, Coats KH, Swisher E, Stray SM, Higgins J. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA. 2006;295:1379–88.
Cavaciuti E, Lauge A, Janin N, Ossian K, Hall J, StoppaLyonnet D. Cancer risk according to type and location of ATM mutation in ataxia-telangiectasia families. Genes Chromosomes Cancer. 2005;42:1–9.
Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230:29–41.
Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006;25:5898–905.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Schlam I, Nunes R, Lynce F. Profile of margetuximab: evidence to date in the targeted treatment of metastatic HER2-positive breast cancer. Onco Targets Ther. 2022;15:471–8.
Delgado E. Treatment updates for metastatic breast cancer. US Pharm. 2012;46:42–6.
Meesters RJ, Voswinkel S. Bioanalytical method development and validation: from the USFDA 2001 to the USFDA 2018 guidance for industry. J Appl Bioanal. 2018;4(3):67–73.
Moein MM, El Beqqali A, Abdel-Rehim M. Bioanalytical method development and validation: critical concepts and strategies. J Chromatogr B. 2017;1043:3–11.
Nashik Sanap S, Sankar Bhatta R, Gupta N, Gauttam VK, Gupta S. Development and validation of an LC-MS/MS method for the assessment of isoxazole, a bioactive analogue of curcumin in rat plasma: application to a pharmacokinetic study. J Chromatogr B. 2022;1212:123488.
Jaochico A, Sangaraju D, Shahidi-Latham SK. A rapid derivatization-based LC-MS/MS method for quantitation of short chain fatty acids in human plasma and urine. Bioanalysis. 2019;11:741–53.
Eswarudu MM, Rao AL, Vijay K. Development and validation of a LC-MS/MS method for simultaneous quantification of ivabradine and metoprolol in rat plasma. J Pharmacol Toxicol Methods. 2022;116:107186.
Hantash J, Smidt ML, Bowsher RR. The development, optimization and validation of an ELISA bioanalytical method for the determination of cetuximab in human serum. Anal Methods. 2009;1(2):144–8.
Dadge SD, Tiwari N, Husain A, Verma S, Agarwal A, Garg R, Rath SK, Shanker K, Gayen JR. Simultaneous estimation of five biomarkers of neuroprotective herb Ashwagandha NMITLI-118R AF1 in rat plasma and brain using LC-ESI-MS/MS: application to its pharmacokinetic and stability studies. J Chrom B. 2023;1228:123834.
Vezzelli A, Verzè S, Morbioli L, Solazzo L, Greco A, Benetti C, Cenacchi V, Breda M. Development and validation of a bioanalytical method for the quantification of CHF6550 and its metabolite (CHF6671) in rat plasma and lung homogenate using LC–MS/MS. J Chrom B. 2023;1222:123678.
Divya Bhargavi P, Lolla S, Sugunan S, Shiva Gubbiyappa K, Ali Khan A, Alanazi AM, Vijay Nayak B. The simultaneous quantification of sitagliptin and irbesartan in rat plasma using the validated LC-MS/MS method is applied to a pharmacokinetic study. J Chrom B. 2023;1221:123677.
Awosemo O, Neelakantan H, Watowich S, Ma J, Wu L, Chow DSL, Liang D. Development & validation of LC–MS/MS assay for 5-amino-1-methyl quinolinium in rat plasma: application to pharmacokinetic and oral bioavailability studies. J Pharm Biomed Anal. 2021;204:114255.
Acknowledgements
The authors are thankful to the management of GITAM (Deemed to be University), Visakhapatnam, Andhra Pradesh, India, for providing necessary facilities and M.V.V.S Murthi fellowship grants to carry out the research work.
Author information
Authors and Affiliations
Contributions
Pridhvi Krishna Gaddey: methodology, validation, formal analysis, investigation, conceptualisation, writing—original draft, writing—review and editing; Raja Sundararajan: methodology, supervision.
Corresponding author
Ethics declarations
Ethical Declaration
All procedures involving animals were in compliance with the CPCSEA guidelines and ethical approval was granted by the Institute of Animals Ethics Committee (Reg. No. 1250/PO/RcBi/S/09/CPCSEA).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gaddey, P.K., Sundararajan, R. Liquid Chromatography Tandem Mass Spectrometric Method for Quantification of Margetuximab in Rat Plasma and Application to a Pharmacokinetic Study. AAPS PharmSciTech 25, 33 (2024). https://doi.org/10.1208/s12249-024-02755-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02755-4