Abstract
The emergence of novel respiratory infections (e.g., COVID-19) and expeditious development of nanoparticle-based COVID-19 vaccines have recently reignited considerable interest in designing inhalable nanoparticle-based drug delivery systems as next-generation respiratory therapeutics. Among various available devices in aerosol delivery, dry powder inhalers (DPIs) are preferable for delivery of nanoparticles due to their simplicity of use, high portability, and superior long-term stability. Despite research efforts devoted to developing inhaled nanoparticle-based DPI formulations, no such formulations have been approved to date, implying a research gap between bench and bedside. This review aims to address this gap by highlighting important yet often overlooked issues during pre-clinical development. We start with an overview and update on formulation and particle engineering strategies for fabricating inhalable nanoparticle-based dry powder formulations. An important but neglected aspect in in vitro characterization methodologies for linking the powder performance with their bio-fate is then discussed. Finally, the major challenges and strategies in their clinical translation are highlighted. We anticipate that focused research onto the existing knowledge gaps presented in this review would accelerate clinical applications of inhalable nanoparticle-based dry powders from a far-fetched fantasy to a reality.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The burden of various respiratory conditions (e.g., coronavirus disease 2019 (COVID-19), lung cancer, tuberculosis, chronic obstructive pulmonary disease (COPD), etc.) has risen sharply over the past decades, warranting the development of highly effective and safe respiratory therapeutics. Pulmonary delivery of respiratory therapeutics is preferred over systemic (e.g., intravenous or oral) administration to achieve localized delivery of drugs to the lungs for improved treatment efficacy and reduced dose requirements, while simultaneously limiting off-target drug distribution and thereby reducing systemic adverse effects [1].
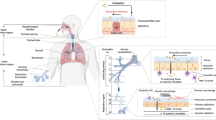
Recent advances in nanotechnology have attracted significant interest in utilizing nanoparticles (herein defined as particles < 1000 nm for purposes of this review) as carriers for pulmonary drug delivery. Compared to conventional inhalable formulations where the drug is present either in solution or suspension form (e.g., as formulations for use in nebulizers) or as micronized dry particles in conventional inhalable dry powder formulations, inhalable nanoparticle-based drug delivery systems possess multiple unique merits (Fig. 1) [2]. Firstly, nanoparticles can be decorated with ligands (e.g., antibodies, peptides, etc.) on the nanoparticle surface to target specific cell types within the lungs, e.g., lung tumor cells [3]. Secondly, while microparticles or sub-micron particles in the size range of 0.5 – 3 µm are readily phagocytosed by alveolar macrophages [4], nanoparticles below < 300 nm can escape macrophage uptake and protect the drug cargo from enzymatic degradation [5]. Thirdly, nanoparticles can improve drug uptake into cells compared to free drugs by various endocytosis-based pathways [6]. Fourthly, sustained release or even controlled release of drugs is possible via modulation of the nanocarrier properties [7]. Finally, and most notably, nanoparticles can translocate from the alveoli to the systemic circulation while microparticles cannot, thereby providing an alternative method for systemic delivery of nanoparticles without the need for invasive intravenous administration [8]. This feature is particularly useful for conditions with both lung and systemic manifestations (e.g., metastasized lung cancers, tuberculosis, etc.).
An important consideration for pulmonary delivery of nanoparticles is the choice of the aerosol-generating device. Various types of devices are currently commercially used for generating inhalable aerosols, including nebulizers, pressurized metered-dose inhalers (pMDIs), soft-mist inhalers, and dry powder inhalers (DPIs). However, the high surface energy of nanoparticles, particularly in suspension form, would promote their aggregation by Ostwald ripening and recrystallization [9]. DPIs are therefore more suitable for the delivery of nanoparticles by oral inhalation due to their greater physical stability. DPIs are also more portable compared to nebulizers and eliminate the need for hand-breath coordination with pMDIs, which makes them particularly attractive for long-term treatment of chronic pulmonary diseases [10]. This review thus focuses on inhalable nanoparticle-based dry powder formulations, i.e., powder formulations with nanoparticles either adsorbed onto the surface of a carrier powder, agglomerated into powder, or dispersed within the powder matrix (see Subsection “Formulation Strategies”) which have suitable physicochemical properties for oral inhalation. Inhalable nanoparticle-based powders differ from conventional inhalable powder formulations (e.g., carrier-blend particles, large porous particles, etc. [11]) in that for conventional inhalable powder formulations drug particles are present in micronized form. Upon inhalation, the micronized drug in conventional inhalable powder formulations dissolve in lung lining fluid as drug molecules, while for inhalable nanoparticle-based powders the powder redisperses into primary nanoparticles upon contact with lung lining fluid, which is critical to the unique merits of inhalable nanoparticle-based formulations as discussed above.
Efficient pulmonary deposition of nanoparticles is a pre-requisite for inhalable nanoparticle-based formulations to exert their therapeutic effect. However, direct oral inhalation of nanoparticles (including nano-sized powders) would result in ineffective delivery of nanoparticles to the lungs as particles with aerodynamic diameter (DA) < 1 µm would likely escape impaction and sedimentation and remain suspended in the airways after inhalation before being exhaled [11]. As particles with DA 1 – 5 µm tend to deposit in the deep lungs by sedimentation while those with DA > 5 µm would be trapped in the upper airways by inertial impaction, inhalable nanoparticle-based powders must be precisely engineered to fulfil the particle size requirement for deep lung deposition [11]. Once the powder particles deposit into the lungs, they should readily redisperse into primary nanoparticles upon contact with alveolar lung lining fluid. However, the nanoparticle suspension drying process exerts various stresses (e.g., shear and thermal stress) onto the primary nanoparticles which could damage their integrity, resulting in changes to their size distribution and/or morphology upon redispersion that could significantly affect their biological fate and therapeutic performance [2].
Over the past decades, significant research has been dedicated to overcoming the abovementioned challenges in developing inhalable nanoparticle-based dry powder formulations, i.e., the engineering of nanoparticle-based powders with (1) dA between 1 and 5 µm and (2) satisfactory redispersibility back into primary nanoparticles upon contact with aqueous medium. Despite such efforts, the mainstream of inhalable nanoparticle-based formulations tested in clinical trials and subsequently approved (e.g., Arikayce®) were used in combination with nebulizers [12,13,14,15]. Only few inhalable nanoparticle-based dry powder formulations have entered early-stage clinical trials [16, 17] with none having successfully obtained regulatory approval to the best of our knowledge, indicating that there is a significant translational barrier for these formulations. The main objective of this review is to address the existing gap for inhalable nanoparticle-based dry powder formulations between bench and bedside. We begin with providing an update on formulation and particle engineering techniques to fabricate inhalable nanoparticle-loaded dry powders formulations with relevant literature examples. Considerations in in vitro characterization methodologies to improve the correlation between in vitro performance and biological fate are then discussed. Finally, the major knowledge gaps in their clinical translation and research directions are identified and addressed with our opinion.
Considerations in Development of Inhalable Nanoparticle-Based Dry Powders
The development of inhalable nanoparticle-based dry powders requires the judicious selection of (1) drying adjuvants in the formulation to protect nanoparticles from drying stresses and (2) particle engineering technique to form nanoparticle-loaded dried powder with suitable aerosol performance for deep lung delivery. Various approaches have been attempted in past research, and a summary of such studies is provided in Table I.
Nanoparticle design is the first important consideration in the development of inhalable nanoparticle-based powders. Various types of nanoparticles, e.g., polymeric nanoparticles [83], liposomes [84], solid lipid nanoparticles [7], mesoporous silica nanoparticles [85], etc., have been extensively researched as nanocarriers for pulmonary delivery, with polymeric and lipid nanoparticle systems being the most popular types of nanocarrier investigated in inhalable nanoparticle-based powders (Table I) due to their relative abundance of clinical safety data. Extensive reviews on the respective advantages, limitations, and design considerations of different types of nanoparticles are available elsewhere [7, 83,84,85]; in this review, the focus is mainly on the formulation and particle engineering strategies for fabricating inhalable nanoparticle-based powders.
Formulation Strategies
While various terminologies have been used to describe inhalable nanoparticle-loaded dry powders, they can generally be divided into three types: Nano-embedded microparticles, nanoagglomerate microparticles, and nanoparticle-carrier systems. A schematic diagram of these designs and their dispersion mechanisms within the respiratory tract is depicted in Fig. 2, and their pharmaceutical properties are summarized in Table II.
Nano-Embedded Microparticles
Nano-embedded microparticles (also known as nano-in-microparticles) consist of drug-loaded nanoparticles embedded or dispersed within a micron-sized matrix, produced by drying of nanosuspensions with dissolved bulking or shell-forming agents. During drying, the bulking or shell-forming agent(s) form a matrix to protect nanoparticles from physical stresses. The morphology of the produced particles is generally spherical in shape with observable dispersion of nanoparticles within the matrix (Fig. 3a). Upon deposition into the lungs, the matrix structure degrades within the lung lining fluid to release nanoparticles (Fig. 2a). Due to the high proportion of excipients required by mass to form the matrix, the overall drug loading is generally low. This strategy therefore is more suitable for the delivery of nanoparticles encapsulating highly potent drugs.
Disaccharides (e.g., lactose, trehalose) and alcohol sugars (e.g., mannitol) are frequently used as bulking or shell-forming agents, with lactose and mannitol being the most common choices as regulatory authorities have approved their use in oral inhalation products. It is worth noting that lactose is unsuitable for protein-carrying formulations as its reducing properties could trigger undesired Mailliard reaction [10]. Furthermore, the high hygroscopicity of lactose may induce significant moisture sorption and thus deteriorate aerosol performance. For inhalable nanoparticle-based powders, high hygroscopicity may also induce destabilization of nanoparticles (e.g., by Ostwald ripening) upon contact with absorbed moisture. This could be overcome with the incorporation of dispersion enhancers, which are usually hydrophobic amino acids (e.g., leucine [87] and phenylalanine [26]). Mannitol is an alternative that can circumvent issues associated with lactose as it is non-reducing and relatively less hygroscopic. Mannitol also is more suitable for patients with diabetes mellitus as it is passively absorbed into the body [88], and its mucolytic properties make it a preferable carrier for conditions involving excessive mucus production (e.g., cystic fibrosis, for which it is an FDA-approved treatment to improve pulmonary function [89]). However, some patients may be hypersensitive to mannitol, and a mannitol tolerance test is recommended prior to treatment initiation [90]. Furthermore, its long-term safety remains unestablished due to its only recent approval by the US FDA.
Nanoagglomerate Microparticles
Nanoagglomerate (also known as nanoaggregate, nano-matrix [67], or Trojan [91]) microparticles consist of nanoparticles agglomerated with each other in a controlled manner after drying. The morphology of inhalable nanoagglomerate powder formulations is normally either hollow or porous, facilitating its low particle effective density (ρeff) for superior aerosolization (Fig. 3b). Upon dispersion as aerosols from the DPI and deposition into the lungs, the nanoagglomerate microparticles would redisperse into primary nanoparticles to exert their therapeutic effects (Fig. 2b).
Nanoagglomerate microparticles are engineered by drying nanosuspensions with dissolved protectants and optional dispersion enhancers (e.g., leucine) in low quantities. The protectant(s) not only protect primary nanoparticles from structural damage due to drying stresses, but also form “bridges” between agglomerated nanoparticles to facilitate redispersion of primary nanoparticles upon contact with the lung lining fluid [48]. Similar to nano-embedded microparticles, lactose and mannitol are the most frequently employed protectants, yet both have their respective drawbacks. Mannitol undergoes recrystallization upon heating/freezing, which not only reduces its protective function, but also further aggravates mechanical stresses exerted on primary nanoparticles during drying. This results in deteriorated aqueous redispersibility of the resultant dry powders [2, 48]. Mannitol may also undergo polymorphic transformations by interacting with nanoparticle stabilizer [46], which may affect the aerosol performance of the resultant dried powder [92]. While lactose remains amorphous throughout the drying process and thus offers stronger protection over mannitol, the resultant powder would likely suffer from poor flowability and aerosol performance as mentioned above. The use of polymers as protectants has emerged as a promising alternative to overcome such drawbacks. For instance, Cheow et al. reported that using polyvinyl alcohol (PVA) as protectant for spray-freeze-drying polycaprolactone (PCL) nanoparticles resulted in dry powder formulations with superior aqueous redispersibility and similar aerosolization characteristics compared to formulations with mannitol as protectant [48], while Wan et al. demonstrated that co-spray-drying itraconazole nanosuspensions with methylcellulose, a gel-forming polymer that undergoes in situ thermal gelation upon heating within a spray dryer to entrap and protect nanoparticles could yield inhalable dry powder with excellent aqueous redispersibility [2]. However, the use of polymers over lactose and mannitol as protectants remains less common (see Table I) as such polymers have not been used as excipients in approved oral inhalation products, and their safety requires further investigation (see Sect. 4.3).
Nanoparticle-Carrier Systems
A less commonly used method to deliver nanoparticles into the lungs as inhalable dry powders is physical adsorption of dried nanoparticles onto the surface of a coarse inert carrier (usually lactose) via either coating or blending. Unlike nano-embedded and nanoagglomerated microparticles where nanoparticle release into the lung lining fluid is based on dissolution of the adjuvant bridges/matrix, physical detachment of the nanoparticles from the micron-sized carrier, which requires a high flow rate that may not be achieved by patients with impaired pulmonary function, is necessary (Fig. 2c) [93]. Furthermore, a large quantity of the carrier relative to nanoparticles is required (i.e., very low drug loading), and the large geometric particle size of the coarse inert carrier results in substantial deposition of powder within the throat by inertial impaction [94], which severely limits nanoparticle deposition in the deep lungs. Therefore, nanoparticle-carrier systems are less preferable compared to nano-embedded and nanoagglomerate microparticles.
Particle Engineering Techniques
The most common nanoparticle-based dry powder formulations (i.e., nano-embedded microparticles and nanoagglomerate microparticles) are engineered by drying of nanosuspension with dissolved drying adjuvants by various drying techniques, with spray drying, spray freeze drying, and freeze drying being the most common techniques employed. This section only serves to provide a brief overview on these techniques and relevant considerations for inhalable nanoparticle-based powders; extensive reviews on the particle engineering of inhalable dry powders are available in the literature [88, 95].
Spray Drying (SD)
Spray drying is the most commonly used method to produce inhalable nanoparticle-based powders (see Table I), in which the feed liquid containing nanosuspension and dissolved drying adjuvants is atomized into droplets and dried with heated gas to result in nanoparticle-based dried powder. The morphology of spray-dried powder normally is hollow and wrinkled or dimpled, which facilitates its low density and therefore appropriate dA for inhalation [96]. Nano-sized powders could also be directly produced using a nano spray dryer for subsequent blending with carriers [97].
The main advantage of spray drying is its facilitation of precise control of particle size and therefore in vitro aerosol performance of inhalable dry powders by manipulating spray drying processing parameters [2]. However, it must be emphasized that the correlation between spray drying parameters and in vitro aerosol performance for inhalable nanoparticle-based powders is also formulation dependent, and thus the results could deviate from expected trends. For example, while an increase in feed pump rate would be expected to result in larger droplet (and therefore particle) size, Wan et al. reported absence of a clear trend between the feed pump rate and the geometric median diameter/in vitro aerosol performance metrics of spray dried itraconazole nanoagglomerate microparticles [2]. As both processing and formulation parameters could significantly affect the aqueous redispersibility of inhalable nanoparticle-based powder formulations [2], rational optimization of these parameters, for example via design of experiments (DoE), is warranted to produce inhalable nanoparticle-based powders with both good redispersibility and aerosol performance [98]. Another unique merit of spray drying is its shorter processing time, which can minimize the risk of causing colloidal instability issues of nanoparticles during drying. Spray drying is also a scalable and continuous process that is highly suitable for pre-clinical and clinical development of relatively costly nanoparticle-based DPI products.
One key disadvantage of spray drying is that it is less preferred for heat-sensitive materials. These materials not only include heat-labile drugs and biologics but also low melting point polymers, e.g., polycaprolactone and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) [2, 99] which are commonly used as nanocarriers and stabilizers. A novel solution to overcome this issue is the use of supercritical fluid-assisted spray drying (SASD), where carbon dioxide (CO2) in supercritical fluid state is mixed and solubilized within the feed liquid prior to spray drying upon which the CO2 vaporizes. The spray drying process operates at a lower drying temperature (typically < 60°C [95]), thereby minimizing the thermal stress on the nanoparticles and energy input. The SASD technique has been successfully applied to the drying of 5(6)-carboxyfluorescein-encapsulating liposomes into inhalable nano-embedded microparticles with good aqueous redispersibility [19]. Another disadvantage of spray drying is its relatively low yield as 30–50% (w/w) of dry powders would be retained in the cyclone or other components of the spray dryer, with collection efficiency particularly poor for particles < 2 µm [100]. To overcome this limitation, nano spray drying has been developed where droplets are produced using a vibrational mesh and fine particles are collected electrostatically [97]. The nano spray drying technique not only improves sample recovery and yield (especially particles < 2 µm) but also is capable to produce powders with superior aerosol performance relative to conventional spray drying [97].
Spray Freeze Drying (SFD)
Unlike spray drying which involves heat input, spray freeze drying involves the freezing of droplets atomized from an atomizer or a spray nozzle using a cryogen and lyophilization of the frozen droplets in a freeze dryer. Similar to spray drying, particle size control in spray freeze drying could be achieved by controlling the processing and formulation parameters. However, there are limited reports on how various processing parameters (e.g., primary drying temperature) as well as their interactions could influence the aqueous redispersibility of spray-freeze-dried inhalable nanoparticle-based dry powders. More studies are warranted to guide future development of these formulations.
Head-to-head studies comparing spray drying versus spray freeze drying revealed superior in vitro aerosol performances of inhalable nanoparticle-based powders produced by the latter technique [43,44,45, 48], possibly due to the highly porous nature of the particles produced upon interstitial sublimation of water from the frozen droplets. However, whether spray freeze drying is superior to spray drying with regard to aqueous redispersibility of these formulations remains controversial, with multiple head-to-head studies reporting that spray freeze drying is superior [43, 44, 48] while Yu et al. reported an opposite trend, attributing such observation to formation of ice crystals during freezing [45]. Obviously, spray freeze drying is more suitable for heat sensitive nanoparticles, and the production yield is significantly higher compared to spray drying. However, spray freeze drying suffers from low throughput due to the long lyophilization process, and significant challenges remain in the translation of the spray freeze drying technique into production scale. This is likely why despite the abovementioned merits, spray freeze drying remains less evaluated for production of inhalable nanoparticle-based powders compared to spray drying (Table I).
Freeze Drying (FD)
Freeze drying involves the freezing of nanosuspensions with cryoprotectants followed by sublimation of the solvent under low pressure and temperature (primary drying), and then heating to remove the remaining solvent content (secondary drying). As freeze drying does not involve spraying of the feed liquid into droplets, particle size control of dry powders is more difficult compared to spray drying and spray freeze drying techniques. Indeed, freeze drying of nanosuspensions often results in powder with inferior in vitro aerosol performance due to the heterogeneous particle size distribution. Furthermore, irreversible aggregation may occur during the freezing of nanosuspensions in bulk, resulting in dry powder with poor aqueous redispersibility [101]. Combined with the inherit disadvantage of long processing times, freeze drying is less common in the particle engineering of inhalable nanoparticle-based dry powders.
Characterization and Quality Attributes for Inhaled Dry Powder
As with other dosage forms, the quality of DPI formulations must be carefully evaluated for their therapeutic potential. Table III summarizes the critical quality attributes and related characterization techniques of DPI formulations.
While the critical quality attributes listed in Table III are universal to all inhalable dry powder formulations, attention should be paid to certain attributes for inhalable nanoparticle-based powders. Drug loading of the powder is a major concern as a low drug loading would result in the patient being required to inhale a high quantity of powder per dose for therapeutic effect which may not be feasible in the clinical setting. As presented in Table I, certain inhalable nanoparticle-based powder formulations require a high portion of excipient (nanoparticle loading of < 10% (w/w) powder mass) for sufficient protection of nanoparticles. During drug development, the formulation should be optimized such that only a minimal amount of excipient is required to provide satisfactory nanoparticle protection during drying. Inhalable nanoparticle-based powders should also be non-hygroscopic to minimize moisture sorption. Finally, certain processes used in fabricating nanosuspensions (e.g., anti-solvent precipitation) require the use of organic solvent which pose a concern of toxicity upon inhalation. Residual solvent content thus must be checked with references to pharmacopoeial and other regulatory guidelines to avoid potential safety issues.
In addition to routine characterizations, extra studies should be carried out to demonstrate the clinical utility of inhalable nanoparticle-based drug delivery systems, as stated below:
Redispersibility and Stability of Redispersed Primary Nanoparticles
To fully take advantage of nanotechnology for pulmonary drug delivery, inhaled nanoparticle-based powders must readily redisperse into primary nanoparticles upon contact with lung lining fluid. The redispersed primary nanoparticles should retain a similar particle size distribution and morphology to that prior to drying. The redispersibility index (RdI) is used as a simple indicator to evaluate powder redispersibility in an aqueous medium [101]. It is defined as the ratio of particle size of primary nanoparticles after powder reconstitution in aqueous media (Sf) over the size of nanoparticles prior to drying (Si) (i.e., Sf/Si). A RdI of unity indicates perfect redispersibility without particle size change. An acceptable Sf/Si is considered to be within the range of 0.7–1.3 [2]. A review of literature (Table I) indicates that there is a lack of report on RdI and Sf of inhaled nanoparticle-based dry powder formulations in the scientific community, and thus their clinical potential remains unknown.
There is a degree of variability in the scientific literature on the methodology in determining aqueous redispersibility of inhalable nanoparticle-based powders. Firstly, various methods to disaggregate the nanoparticles upon addition of powder to the aqueous medium were adopted, including sonication [104], hand agitation [46], etc. However, such high-intensity processes do not mimic the actual redispersion of powder in the human body as powders come into contact with lung lining fluid without external mechanical agitation. It is recommended to adopt a spontaneous aqueous re-dispersion method, i.e., adding the powder to the reconstitution medium and let the suspension set until no visible particulates are observed prior to sizing. Secondly, there is significant variation in the reconstitution medium used. While reconstitution in pure water is most straightforward to assess redispersibility and has been adopted by many studies [2, 43, 49, 61], it may not truly represent the fate of nanoparticle-based powders upon redispersion of primary nanoparticles in lung lining fluid, which has a different pH from pure water and contains various types of soluble phospholipids and proteins [105]. These components may alter nanoparticle stability by complex bio-nano interactions, thereby hindering their intended therapeutic function. Therefore, it is highly recommended that redispersibility studies are performed in both deionized water and biologically relevant media such as stimulated lung fluid (SLF) [105] and phosphate-buffered saline (PBS) [106].
It must be emphasized that redispersibility studies could only detect changes in particle size, and other relevant physicochemical changes could also occur, e.g., drug degradation or changes in particle morphology during the stressful drying processes and subsequent storage. However, only few studies have evaluated these attributes. Suitable techniques should be used to confirm the colloidal and chemical stability of redispersed primary nanoparticles, including but not limited to transmission electron microscopy (TEM) for observation of redispersed nanoparticle morphology and nanoparticle separation techniques (e.g., centrifugal ultrafiltration) to determine drug loading and encapsulation efficiency of redispersed primary nanoparticles [107]. Significant deviations of such properties from the nanosuspension before drying would require further optimization of the formulation (e.g., an increase in protectant amount). The long-term storage stability of the inhalable nanoparticle-based powders should also be assessed according to ICH or relevant pharmacopoeial conditions, and verification of relevant parameters such as redispersibility, aerosol performance, drug loading and encapsulation efficiency, redispersed nanoparticle morphology, etc. As the protein corona is known to alter the colloidal stability of nanoparticles and may affect the results of in vitro cellular or in vivo studies, it is suggested to monitor the colloidal stability of redispersed primary nanoparticles in biological media (e.g., complete cell culture medium) to improve in vitro-in vivo correlation (IVIVC) [106]. Modification of nanocarrier surface (e.g., PEGylation or protein adsorption) could enhance colloidal stability in the biological environment [106].
In Vitro Drug Release
Unlike typical dissolution where drugs are present in molecular form and their concentrations can be directly determined by UV, HPLC, or LC/MS/MS, separation of nanoparticles from release medium should be performed in order to obtain an accurate in vitro drug release profile. Currently, there are no compendial methods for assessing either dissolution profile of inhaled dry powders or in vitro drug release of nanoparticles, resulting in substantial variations in the methodology among literature reports. This not only causes erratic correlations between in vitro release of drug nanoparticles and in vivo pharmacokinetics, but also impedes the systematic head-to-head comparison among the reported systems. To set a stage for IVIVC for inhalable nanoparticle-based powders, several factors should be taken into consideration for the experimental design. Firstly, a bulk sample of powder was directly dispersed into the release medium in most studies on inhalable nanoparticle-based powders. While this is the most convenient approach, only particles with dA < 5 µm can practically reach the lungs. Therefore, it is recommended that the FPF of powders should first be separated from the bulk sample, e.g., using a fast-screening impactor (FSI) [1] or modifying the powder collection procedure of the pharmacopoeial apparatus used to assess in vitro aerosol performance [108], prior to dispersion into release medium. Secondly, as mentioned, the presence of drug in the release medium could either be in dissolved form or as redispersed nanoparticles, and separation of these two forms is needed to avoid overestimating in vitro drug release. The most common methods to determine in vitro nanoparticle drug release include continuous flow, dialysis membrane and sample & separate methods [107], and with continuous flow [30, 46] and dialysis membrane [20, 26] methods most commonly seen in studies on inhalable nanoparticle-based powders. However, each method has their own limitations. The continuous flow methods are costly with complicated experimental setups. Dialysis membrane methods may overestimate release kinetics of drug-loaded polymeric nanoparticle systems due to interactions between the dialysis membrane and nanoparticles [107]. The major challenge for sample & separate method is in the separation of nanoparticles from free drugs after sampling. While Weng et al. [107] have developed a new sample & separate method to determine drug release from polymeric nanoparticles with higher accuracy and precision, the appropriateness of this technique for inhalable nanoparticle-based powders requires further investigation. Finally, there is significant variation in the volume of the release medium across literature. Some studies have utilized a very high volume (e.g., 200 mL [21, 29]) of release medium which significantly deviates from the volume of human lung lining fluid; 10 – 30 mL is generally considered for better correlation with the clinical application of inhalable nanoparticle-based powders [109]. As with redispersibility studies, in vitro drug release studies should be performed in biorelevant media such as PBS or SLF.
In Vitro Aerosol Performance
Examination of the in vitro aerosol performance of nanoparticle-based dry powders is critical for predicting their lung deposition efficiency upon inhalation in vivo. The primary parameters required to be reported include the fine particle fraction (FPF) (normally defined as fraction of particles with dA ≤ 5 µm), the mass median aerodynamic diameter (MMAD), and the geometric standard deviation (GSD). Several types of pharmacopoeial apparatuses are available for this purpose, including multi-stage liquid impingers (MSLI), Andersen cascade impactor (ACI), Next Generation Impactor (NGI), etc. The NGI is often preferred over other apparatuses as it offers superior resolution of the aerodynamic particle size distribution with minimal inter-stage overlap across a wide range of airflow rates (30 – 100 L/min) [110].
Experimental considerations in in vitro aerosol performance testing of any dry powder formulations include the choice of inhaler device used and the range of airflow rate tested. For passive inhalers (the most common type of inhalers available on the market), effective powder dispersion from the device relies on the achievement of a sufficient airflow rate (Q) across the device, requiring patients to generate a sufficient inspiratory effort or pressure drop (ΔP, usually 1 – 6 kPa) against the intrinsic resistance (R) of the device (i.e., \(\sqrt{\Delta P}=Q\times R\)) [111]. Various commercial inhaler devices with different intrinsic resistances are available and classified into low-resistance (e.g., Breezhaler®, Aerolizer®), medium-resistance (e.g., Ellipta®, Accuhaler®), and high-resistance (e.g., Handihaler®) devices [112]. While low-resistance devices require a smaller inspiratory effort to achieve higher flow rates across the device, they simultaneously have a higher flow rate requirement to disperse the formulations compared to high-resistance devices [111]. It is observed that most studies on inhalable nanoparticle-based powders only tested the formulations using a single type of inhaler as proof-of-concept (Table I). However, during commercial development, it is recommended to evaluate the in vitro aerosol performance of the formulation using a range of inhaler devices to identify the device with best aerosolization efficiency of the formulation.
Furthermore, optimal flow rates for different devices are established based on their commercial formulations, which may not apply when used with other formulations, especially since the morphology of dry powders affects their dependence on airflow rate for effective dispersion [113]. However, our literature review (Table I) finds that most studies on inhalable nanoparticle-based powders only performed in vitro aerosol performance testing using a fixed flow rate, which do not take account of the large inter-patient variability of inspiratory effort across patients of varying pulmonary function. During pre-clinical development of an inhalable nanoparticle-based DPI product, it is suggested that a wide range of inspiratory flow rates should be covered, with a careful consideration of the diseases and patient conditions. Most patients across various respiratory conditions (e.g., asthma [114], COPD [114], pulmonary arterial hypertension [115], and cystic fibrosis [116]) can achieve a peak inspiratory flow rate in the range of approximately 40 – 100 L/min using most commercially available passive inhaler devices; thus, selection of tested flow rates within this range (e.g., 45, 60, and 90 L/min) is advised. In later stages of pre-clinical development, the use of breathing simulators in impactor testing that mimics actual patient respiratory patterns may result in stronger IVIVC [117]. If significant variations in aerosolization efficiency (e.g., FPF) across varied flow rates or respiratory profiles occur, the formulation should further be optimized.
Challenges and Knowledge Gaps for Clinical Translation
The successful clinical translation of any pharmaceutical product requires the product to demonstrate satisfactory efficacy, safety, and quality. However, thorough evaluation of these attributes for nanomedicine is complicated as there is a lack of standardized regulatory guidance available with great inconsistency among guidelines issued by regulatory agencies across the globe [118]. For inhalable nanoparticle-based powders, unique regulatory issues arise due to their intended route of administration (i.e., oral inhalation). While regulatory challenges for nanomedicines have been summarized concisely in a recent review by Foulkes et al. [118], in this section important knowledge gaps and relevant future research directions to the clinical translation of inhalable nanoparticle-based dry powders are addressed (Fig. 4) to set the stage for future regulatory guidance and successful commercialization.
Manufacturing Scale-up and Process Optimization
A major challenge in the clinical translation of nanomedicines is their reproducible and scalable production, which is particularly complex for inhalable nanoparticle-based dry powders as such requirements are pertinent for both nanosuspension fabrication and drying processes. Conventional nanoparticle fabrication techniques are conducted in batch with significant batch-to-batch variability in nanoparticle properties, particularly particle size [119]. Further variability is often observed during scaling-up of nanoparticle production [120] as changes in production scale likely bring about variations in mass transfer rate and momentum of building blocks that dictate nanoparticle assembly [121]. It is critical to achieve a stringent particle size control of primary nanoparticles as it not only affects their biological fate (see Sect. 4.2) upon redispersion, but also impacts the aerosol performance of the dried powder [122]. Similarly, batch-to-batch variations in aerosol performance of the inhalable dry powder may occur during the drying process, e.g., variations in temperature throughout the drying chamber for (spray) freeze drying [123], and scaling up of (spray) freeze drying remains difficult due to the large equipment footprint and stringent conditions required. Product variability could be reduced by the adoption of the pharmaceutical Quality-by-Design (QbD) approach to enhance product and process understanding, with the ultimate aim of deriving a control strategy for the consistent production of nanomedicines [98]. Batch-to-batch variations and scalability issues may further be minimized by the development of a continuous manufacturing platform combining a continuous nanoparticle fabrication process (e.g., flash nanoprecipitation [FNP] [120] and microfluidic mixers [124]) with a continuous drying operation (e.g., spray drying) [125]. Scale-up (or scale-down) is easily achieved by adjusting the mass flow rate through the platform without the need for large-scale equipment, hence reducing manufacturing costs.
Bio-Fate and Its Correlation with Nanoparticle Physicochemical Properties
While in vitro aerosol performance and redispersibility are major CQAs in the development of inhalable nanoparticle-based dry powders, the physicochemical properties of primary nanoparticles, i.e., particle size, surface charge, and shape, are equally important and should be optimized for maximal therapeutic effect. However, unlike the former where defined requirements exist, there is significant ambiguity in what is considered “optimal” for various nanoparticle physicochemical properties. As shown in Table I, most studies on the inhalable nanoparticle-based powders had a primary nanoparticle size < 300 nm. This size range was generally accepted to be suitable for evading phagocytic clearance and enhancing lung retention of nanoparticles [5]. However, there is few, if any, available studies that systematically investigated particle size effect of inhaled nanoparticles within the 20–200 nm size range of pharmaceutical interest. It is worth mentioning that a smaller particle size within such range does not guarantee superior therapeutic performance. For example, Valsalakumari et al. demonstrated that 120 nm paclitaxel-loaded lipid nanocapsules enhanced cellular uptake into breast cancer cells compared to those with particle size of 50 nm and 90 nm [126], and Weng et al. reported that 40 nm and 150 nm cholecalciferol-loaded nanoparticles showed greater lung deposition compared to those with size between 60 and 125 nm [127]. Similarly, there remains a lack of understanding on the effect of nanoparticle surface charge on the bio-fate of inhaled nanoparticles. While studies have demonstrated that cationic nanoparticles may induce superior cell uptake into lung epithelial cells compared to their anionic and neutral counterparts [128], others have shown that neutral nanoparticles can more effectively penetrate the mucus layer [129]. More studies are needed to establish the correlation between nanoparticle physicochemical properties (including individual and interactive effects) and their biological fates and therefore the “optimal” nanoparticle size and surface charge for maximized therapeutic effect.
Pulmonary Toxicology of Inhalable Nanoparticle-Based Dry Powders
Toxicity has been cited as another major hurdle in bench-to-bedside translation of nanomedicines, especially since ultrafine inorganic particles (e.g., titanium dioxide, gold nanoparticles, etc.) have well-established toxicological effects when inhaled, influenced by their physicochemical properties (e.g., particle size, surface charge, etc.) [130]. However, such results may not be translatable to organic nanoparticles which are the mainstay of inhalable nanoparticle-based dry powders. Nevertheless, novel polymers or lipids are sometimes used to fabricate these organic nanoparticles without thorough evaluation of its biocompatibility and biodegradability when delivered by oral inhalation, significantly hindering their clinical translation. Similarly, various polymers (e.g., PVA, methylcellulose) have not been approved as excipients for oral inhalation despite their potential merits over FDA-approved excipients such as lactose and mannitol as protectants in nanoagglomerate microparticle formulations. While in vitro cellular studies have generally demonstrated low cytotoxicity of inhalable nanoparticle-based dry powders in human pulmonary cell lines, limited in vivo studies investigating the safety of inhalable nanoparticle-based dry powders (especially under chronic exposure) are available, despite the fact that most of the dry powder formulations reported are intended for the treatment of chronic respiratory conditions. As such, research efforts should be dedicated to proper characterization of toxicological profiles of nanoparticle-based dry powder formulations (at both in vitro and in vivo levels) and effects of organic nanoparticle properties (e.g., particle size) on their pulmonary toxicity to ensure their safety in clinical practice.
Articulation of Preclinical Data and Clinical Consideration
The prediction of human in vivo behavior of inhaled nanoparticle-loaded dry powders from preclinical data is critical to their successful clinical translation. As only limited clinical trials have been conducted to-date on inhalable nanoparticle-based powders, there is lack of in vitro and clinical data available for establishing a robust IVIVC, and data available mostly concern nanoparticle-carrier systems. Nevertheless, currently available data suggest a good correlation between in vitro cascade impactor testing and clinical lung deposition data. For example, Bhavna et al. reported a higher respiratory fraction of nano-sized salbutamol-lactose blend over micronized salbutamol-lactose blend (45.2 ± 5.2% vs. 31.3 ± 3.1%) as measured with ACI, which corresponded to a greater deposition of salbutamol in the lungs (64.1 ± 3.7% vs. 28.3 ± 5.2%) as measured using scintigraphy [131]. A similar trend was reported by Kumar et al. for an inhalable edetate calcium sodium (Ca-Na2EDTA) nanoparticle formulation compared to its micronized counterpart [17].
The major challenge in establishing IVIVC for inhalable nanoparticle-based powders is developing preclinical models that accurately reflect the fate of nanoparticle-based powders after oral inhalation, i.e., deposition of powders within the lungs, redispersion of nanoparticles in lung lining fluid (see subsection “Redispersibility and Stability of Redispersed Primary Nanoparticles”), and subsequent absorption and clearance of nanoparticles from the respiratory tract. For lung deposition, cascade impactors are known to have at best modest correlation with in vivo lung deposition data due to differences in “mouth-throat” geometry and inhalation profiles [132]. IVIVC could be improved by employing realistic “mouth-throats” connected to cascade impactors or 3D-printed lung models and simulated breathing profiles [117, 133], yet the selection of suitable anatomical “mouth-throats” and representative inhalation profiles remains to be controversial. Alternatively, in silico modeling by computational fluid dynamics (CFD) is a trendy strategy in predicting in vivo deposition, though they are more technically demanding compared to in vitro methods [134].
For conventional orally inhaled formulations, the absorption and clearance of dissolved drug molecules are assessed in vitro by cell cultures to establish correlation with pharmacokinetic or pharmacodynamic data [135]. However, the study of absorption and clearance of redispersed drug-loaded nanoparticles within the respiratory tract is far more complicated compared to conventional orally inhaled formulations by virtue of their different (or mixed) cellular uptake and clearance mechanisms and the co-presence of drug-loaded nanoparticles and free drugs within the lung lining fluid and cellular environment. Furthermore, animal models may overestimate therapeutic merits of nanoparticles due to interspecies differences in physiology, the enhanced permeability and retention (EPR) effect being the most notable example [136]. It is hard to assess the enhancement in pharmacokinetics or pharmacodynamics of inhaled drug-loaded nanoparticles relative to a conventional inhaled formulation and thereby predict a suitable in vivo dose. With advances in tissue engineering and 3D printing, 3D-bioprinted lung models may serve as a practical tool for in vitro evaluation of inhaled nanoparticle-based dry powders by mimicking the complexity of the human lung physiology and patient’s conditions [135].
Future Perspectives and Conclusions
There is a gradual shift from small molecules to biologics in new drug development due to the high specificity of biologics. Based on the recent success of mRNA vaccines in COVID-19 prophylaxis, the development of inhalable formulations for treatment of respiratory conditions has become an area of active research [10]. Nanoparticle-based formulations play an important role in pulmonary delivery of biologics as they can protect biologics from enzymatic degradation and offer additional therapeutic benefits. For example, encapsulating nucleic acid therapeutics (e.g., siRNA) in nanoparticles has been shown to enhance their cellular uptake and transfection efficiency [72]. Recent research has led to the successful development of inhalable nanoparticle-based dry powders for biologics that could effectively retain the integrity and activity of the encapsulated biologic (Table I). However, it is found that the nano-embedded microparticle strategy is most often employed for these purposes. Combined with their low encapsulation efficiency and drug loading within nanoparticles, inhalation of a large quantity of powder would be required to deliver optimal therapeutic doses which results in high costs and low treatment compliance. There is few, if any, literature available, regarding the adoption of nanoagglomerate microparticles for delivery of biologics, and it could be a propitious option to deliver nano-encapsulated biologics with the least amount of dry powder necessary and a lower requirement of inspiratory flow rates.
In conclusion, the emergence of novel respiratory infections (e.g., COVID-19) and rising prevalence of chronic respiratory conditions have sparked considerable interests in the development of new therapeutics. Formulating therapeutic agents as inhalable nanoparticle-based dry powders is a niche for effective drug delivery into the diseased lungs, combining the merits of pulmonary drug delivery and nanotechnology. Despite precedent successful approval of inhalable nanoparticle nebulized formulations and research efforts dedicated to their formulation development and particle engineering, there still exists knowledge gaps required to be filled for successful clinical translation. This review not only summarizes the current state-of-the-art fabrication of inhalable nanoparticle-based dry powders, but also provides directions to pave the path toward their successful clinical translation. With a suitable biorelevant characterization and comprehensive understanding on the correlation between the physicochemical properties and in vitro performance of inhaled nanoparticles with their clinical response, it is anticipated that the use of inhalable nanoparticle-based dry powders in clinical practice will soon no longer be a far-fetched fantasy but a reality.
Data Availability
No datasets were generated or analysed during the current study.
References
Liao Q, Yip L, Chow MYT, Chow SF, Chan HK, Kwok PCL, et al. Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int J Pharm. 2019;560:144–54. https://doi.org/10.1016/j.ijpharm.2019.01.057.
Wan KY, Weng J, Wong SN, Kwok PCL, Chow SF, Chow AHL. Converting nanosuspension into inhalable and redispersible nanoparticles by combined in-situ thermal gelation and spray drying. Eur J Pharm Biopharm. 2020;149:238–47. https://doi.org/10.1016/j.ejpb.2020.02.010.
Mangal S, Gao W, Li T, Zhou QT. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol Sin. 2017;38(6):782–97. https://doi.org/10.1038/aps.2017.34.
Osman N, Kaneko K, Carini V, Saleem I. Carriers for the targeted delivery of aerosolized macromolecules for pulmonary pathologies. Expert Opin Drug Deliv. 2018;15(8):821–34. https://doi.org/10.1080/17425247.2018.1502267.
Dandekar P, Venkataraman C, Mehra A. Pulmonary targeting of nanoparticle drug matrices. J Aerosol Med Pulm Drug Deliv. 2010;23(6):343–53. https://doi.org/10.1089/jamp.2009.0784.
Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. https://doi.org/10.1016/j.addr.2019.04.008.
Leong EWX, Ge R. Lipid nanoparticles as delivery vehicles for inhaled therapeutics. Biomedicines. 2022;10(9). https://doi.org/10.3390/biomedicines10092179.
Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circ. 2002;105(4):411–4. https://doi.org/10.1161/hc0402.104118.
D’Addio SM, Prud’homme RK. Controlling drug nanoparticle formation by rapid precipitation. Adv Drug Deliv Rev. 2011;63(6):417–26. https://doi.org/10.1016/j.addr.2011.04.005.
Liang W, Pan HW, Vllasaliu D, Lam JKW. Pulmonary delivery of biological drugs. Pharmaceutics. 2020;12(11). https://doi.org/10.3390/pharmaceutics12111025.
Ke WR, Chang RYK, Chan HK. Engineering the right formulation for enhanced drug delivery. Adv Drug Deliv Rev. 2022;191:114561. https://doi.org/10.1016/j.addr.2022.114561.
Khan O, Chaudary N. The use of amikacin liposome inhalation suspension (Arikayce) in the treatment of refractory nontuberculous mycobacterial lung disease in adults. Drug Des Devel Ther. 2020;14:2287–94. https://doi.org/10.2147/DDDT.S146111.
Haworth CS, Bilton D, Chalmers JD, Davis AM, Froehlich J, Gonda I, et al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med. 2019;7(3):213–26. https://doi.org/10.1016/S2213-2600(18)30427-2.
ClinicalTrials.gov: Safety, tolerability and pharmacokinetics of inhaled nanoparticle formulation of remdesivir (GS-5734) and NA-831 (NEUROSIVIR). https://clinicaltrials.gov/ct2/show/NCT04480333 (2020). Accessed 28 Nov 2022.
ClinicalTrials.gov: Inhaled nanosilver study. https://clinicaltrials.gov/ct2/show/NCT02408874 (2019). Accessed 28 Nov 2022.
Ali R, Jain GK, Iqbal Z, Talegaonkar S, Pandit P, Sule S, et al. Development and clinical trial of nano-atropine sulfate dry powder inhaler as a novel organophosphorous poisoning antidote. Nanomed. 2009;5(1):55–63. https://doi.org/10.1016/j.nano.2008.07.001.
Kumar N, Soni S, Jaimini A, Ahmad FJ, Bhatnagar A, Mittal G. Edetate calcium disodium nanoparticle dry powder inhalation: a novel approach against heavy metal decorporation. Int J Pharm. 2011;416(1):376–83. https://doi.org/10.1016/j.ijpharm.2011.06.038.
Zendehdel Baher S, Yaqoubi S, Asare-Addo K, Hamishehkar H, Nokhodchi A. Dry powder formulation of simvastatin nanoparticles for potential application in pulmonary arterial hypertension. Pharmaceutics. 2022;14(5). https://doi.org/10.3390/pharmaceutics14050895.
Costa C, Nobre B, Matos AS, Silva AS, Casimiro T, Corvo ML, et al. Inhalable hydrophilic molecule-loaded liposomal dry powder formulations using supercritical CO2 – assisted spray-drying. J CO2 Util. 2021;53:101709. https://doi.org/10.1016/j.jcou.2021.101709.
Abdelrady H, Hathout RM, Osman R, Saleem I, Mortada ND. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur J Pharm Sci. 2019;133:115–26. https://doi.org/10.1016/j.ejps.2019.03.016.
Yamasaki K, Kwok PC, Fukushige K, Prud’homme RK, Chan HK. Enhanced dissolution of inhalable cyclosporine nano-matrix particles with mannitol as matrix former. Int J Pharm. 2011;420(1):34–42. https://doi.org/10.1016/j.ijpharm.2011.08.010.
Satari N, Taymouri S, Varshosaz J, Rostami M, Mirian M. Preparation and evaluation of inhalable dry powder containing glucosamine-conjugated gefitinib SLNs for lung cancer therapy. Drug Dev Ind Pharm. 2020;46(8):1265–77. https://doi.org/10.1080/03639045.2020.1788063.
Nemati E, Mokhtarzadeh A, Panahi-Azar V, Mohammadi A, Hamishehkar H, Mesgari-Abbasi M, et al. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. AAPS PharmSciTech. 2019;20(3):120. https://doi.org/10.1208/s12249-019-1334-y.
Thiyagarajan D, Huck B, Nothdurft B, Koch M, Rudolph D, Rutschmann M, et al. Spray-dried lactose-leucine microparticles for pulmonary delivery of antimycobacterial nanopharmaceuticals. Drug Deliv Transl Res. 2021;11(4):1766–78. https://doi.org/10.1007/s13346-021-01011-7.
Party P, Bartos C, Farkas A, Szabo-Revesz P, Ambrus R. Formulation and in vitro and in silico characterization of “nano-in-micro” dry powder inhalers containing meloxicam. Pharmaceutics. 2021;13(2). https://doi.org/10.3390/pharmaceutics13020211.
Farhangi M, Mahboubi A, Kobarfard F, Vatanara A, Mortazavi SA. Optimization of a dry powder inhaler of ciprofloxacin-loaded polymeric nanomicelles by spray drying process. Pharm Dev Technol. 2019;24(5):584–92. https://doi.org/10.1080/10837450.2018.1545237.
Nurbaeti SN, Brillault J, Tewes F, Olivier JC. Sustained-release microparticle dry powders of chloramphenicol palmitate or thiamphenicol palmitate prodrugs for lung delivery as aerosols. Eur J Pharm Sci. 2019;138:105028. https://doi.org/10.1016/j.ejps.2019.105028.
Porsio B, Lentini L, Ungaro F, Di Leonardo A, Quaglia F, Giammona G, et al. Inhalable nano into micro dry powders for ivacaftor delivery: the role of mannitol and cysteamine as mucus-active agents. Int J Pharm. 2020;582:119304. https://doi.org/10.1016/j.ijpharm.2020.119304.
Hu X, Yang F, Liao Y, Li L, Zhao G, Zhang L. Docetaxel-loaded cholesterol-PEG co-modified poly (n-butyl) cyanoacrylate nanoparticles for antitumor drug pulmonary delivery: preparation, characterization, and in vivo evaluation. Int J Nanomed. 2020;15:5361–76. https://doi.org/10.2147/IJN.S249511.
Honmane S, Hajare A, More H, Osmani RAM, Salunkhe S. Lung delivery of nanoliposomal salbutamol sulfate dry powder inhalation for facilitated asthma therapy. J Liposome Res. 2019;29(4):332–42. https://doi.org/10.1080/08982104.2018.1531022.
Zhong Q. Co-spray dried mannitol/poly(amidoamine)-doxorubicin dry-powder inhaler formulations for lung adenocarcinoma: morphology, in vitro evaluation, and aerodynamic performance. AAPS PharmSciTech. 2018;19(2):531–40. https://doi.org/10.1208/s12249-017-0859-1.
Abdelaziz MM, Hefnawy A, Anter A, Abdellatif MM, Khalil MAF, Khalil IA. Respirable spray dried vancomycin coated magnetic nanoparticles for localized lung delivery. Int J Pharm. 2022;611:121318. https://doi.org/10.1016/j.ijpharm.2021.121318.
El Baihary D, Osman R, Abdel-Bar HM, Sammour OA. Pharmacokinetic/pulmokinetic analysis of optimized lung targeted spray dried ketotifen-dextran core shell nanocomplexes-in-microparticles. Int J Biol Macromol. 2019;139:678–87. https://doi.org/10.1016/j.ijbiomac.2019.08.011.
Nozohouri S, Salehi R, Ghanbarzadeh S, Adibkia K, Hamishehkar H. A multilayer hollow nanocarrier for pulmonary co-drug delivery of methotrexate and doxorubicin in the form of dry powder inhalation formulation. Mater Sci Eng C Mater Biol Appl. 2019;99:752–61. https://doi.org/10.1016/j.msec.2019.02.009.
Craparo EF, Drago SE, Quaglia F, Ungaro F, Cavallaro G. Development of a novel rapamycin loaded nano- into micro-formulation for treatment of lung inflammation. Drug Deliv Transl Res. 2022;12(8):1859–72. https://doi.org/10.1007/s13346-021-01102-5.
Rezazadeh M, Davatsaz Z, Emami J, Hasanzadeh F, Jahanian-Najafabadi A. Preparation and characterization of spray-dried inhalable powders containing polymeric micelles for pulmonary delivery of paclitaxel in lung cancer. J Pharm Pharm Sci. 2018;21(1s):200s–14s. https://doi.org/10.18433/jpps30048.
Stocke NA, Meenach SA, Arnold SM, Mansour HM, Hilt JZ. Formulation and characterization of inhalable magnetic nanocomposite microparticles (MnMs) for targeted pulmonary delivery via spray drying. Int J Pharm. 2015;479(2):320–8. https://doi.org/10.1016/j.ijpharm.2014.12.050.
Price DN, Stromberg LR, Kunda NK, Muttil P. In vivo pulmonary delivery and magnetic-targeting of dry powder nano-in-microparticles. Mol Pharm. 2017;14(12):4741–50. https://doi.org/10.1021/acs.molpharmaceut.7b00532.
Ungaro F, d’Angelo I, Coletta C, di Villa d’Emmanuele, Bianca R, Sorrentino R, Perfetto B, et al. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Control Release. 2012;157(1):149–59. https://doi.org/10.1016/j.jconrel.2011.08.010.
Sultana S, Talegaonkar S, Ali R, Mittal G, Ahmad FJ, Bhatnagar A. Inhalation of alendronate nanoparticles as dry powder inhaler for the treatment of osteoporosis. J Microencapsul. 2012;29(5):445–54. https://doi.org/10.3109/02652048.2012.655428.
Hu J, Dong Y, Pastorin G, Ng WK, Tan RBH. Spherical agglomerates of pure drug nanoparticles for improved pulmonary delivery in dry powder inhalers. J Nanopart Res. 2013;15(4):1560. https://doi.org/10.1007/s11051-013-1560-2.
Sung JC, Padilla DJ, Garcia-Contreras L, Verberkmoes JL, Durbin D, Peloquin CA, et al. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res. 2009;26(8):1847–55. https://doi.org/10.1007/s11095-009-9894-2.
Ali ME, Lamprecht A. Spray freeze drying for dry powder inhalation of nanoparticles. Eur J Pharm Biopharm. 2014;87(3):510–7. https://doi.org/10.1016/j.ejpb.2014.03.009.
Wang Y, Kho K, Cheow WS, Hadinoto K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid-polymer hybrid nanoparticles. Int J Pharm. 2012;424(1–2):98–106. https://doi.org/10.1016/j.ijpharm.2011.12.045.
Yu H, Teo J, Chew JW, Hadinoto K. Dry powder inhaler formulation of high-payload antibiotic nanoparticle complex intended for bronchiectasis therapy: spray drying versus spray freeze drying preparation. Int J Pharm. 2016;499(1–2):38–46. https://doi.org/10.1016/j.ijpharm.2015.12.072.
Leung SS, Wong J, Guerra HV, Samnick K, Prud’homme RK, Chan HK. Porous mannitol carrier for pulmonary delivery of cyclosporine A nanoparticles. AAPS J. 2017;19(2):578–86. https://doi.org/10.1208/s12248-016-0039-3.
Yu S, Pu X, Ahmed MU, Yu HH, Mutukuri TT, Li J, et al. Spray-freeze-dried inhalable composite microparticles containing nanoparticles of combinational drugs for potential treatment of lung infections caused by Pseudomonas aeruginosa. Int J Pharm. 2021;610:121160. https://doi.org/10.1016/j.ijpharm.2021.121160.
Cheow WS, Ng ML, Kho K, Hadinoto K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: effect of freeze-drying adjuvants. Int J Pharm. 2011;404(1–2):289–300. https://doi.org/10.1016/j.ijpharm.2010.11.021.
Yu H, Tran T-T, Teo J, Hadinoto K. Dry powder aerosols of curcumin-chitosan nanoparticle complex prepared by spray freeze drying and their antimicrobial efficacy against common respiratory bacterial pathogens. Colloids Surf, A. 2016;504:34–42. https://doi.org/10.1016/j.colsurfa.2016.05.053.
Haghighi DM, Faghihi H, Darabi M, Mirmoeini MS, Vatanara A. Spray freeze drying to solidify Nanosuspension of Cefixime into inhalable microparticles. Daru. 2022;30(1):17–27. https://doi.org/10.1007/s40199-021-00426-4.
Changsan N, Sinsuebpol C. Dry powder inhalation formulation of chitosan nanoparticles for co-administration of isoniazid and pyrazinamide. Pharm Dev Technol. 2021;26(2):181–92. https://doi.org/10.1080/10837450.2020.1852570.
Dahmash EZ, Ali DK, Alyami HS, AbdulKarim H, Alyami MH, Aodah AH. Novel thymoquinone nanoparticles using poly(ester amide) based on L-arginine-targeting pulmonary drug delivery. Polym (Basel). 2022;14(6). https://doi.org/10.3390/polym14061082.
Yang Y, Huang Z, Li J, Mo Z, Huang Y, Ma C, et al. PLGA porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv Healthc Mater. 2019;8(23):e1900965. https://doi.org/10.1002/adhm.201900965.
Mukhtar M, Csaba N, Robla S, Varela-Calvino R, Nagy A, Burian K, et al. Dry powder comprised of isoniazid-loaded nanoparticles of hyaluronic acid in conjugation with mannose-anchored chitosan for macrophage-targeted pulmonary administration in tuberculosis. Pharm. 2022;14(8). https://doi.org/10.3390/pharmaceutics14081543.
Puri V, Chaudhary KR, Singh A, Singh C. Inhalation potential of N-Acetylcysteine loaded PLGA nanoparticles for the management of tuberculosis: In vitro lung deposition and efficacy studies. Curr Res Pharmacol Drug Discov. 2022;3:100084. https://doi.org/10.1016/j.crphar.2022.100084.
Sabuj MZR, Dargaville TR, Nissen L, Islam N. Inhaled ciprofloxacin-loaded poly(2-ethyl-2-oxazoline) nanoparticles from dry powder inhaler formulation for the potential treatment of lower respiratory tract infections. PLoS One. 2021;16(12):e0261720. https://doi.org/10.1371/journal.pone.0261720.
Rawal T, Patel S, Butani S. Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur J Pharm Sci. 2018;124:273–87. https://doi.org/10.1016/j.ejps.2018.08.038.
Rawal T, Parmar R, Tyagi RK, Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf B Biointerfaces. 2017;154:321–30. https://doi.org/10.1016/j.colsurfb.2017.03.044.
Debnath SK, Saisivam S, Debanth M, Omri A. Development and evaluation of Chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS One. 2018;13(1):e0190976. https://doi.org/10.1371/journal.pone.0190976.
Shah S, Cristopher D, Sharma S, Soniwala M, Chavda J. Inhalable linezolid loaded PLGA nanoparticles for treatment of tuberculosis: design, development and in vitro evaluation. J Drug Deliv Sci Technol. 2020;60:102013. https://doi.org/10.1016/j.jddst.2020.102013.
Quarta E, Sonvico F, Bettini R, De Luca C, Dotti A, Catalucci D, et al. Inhalable microparticles embedding calcium phosphate nanoparticles for heart targeting: the formulation experimental design. Pharm. 2021;13(11). https://doi.org/10.3390/pharmaceutics13111825.
Mohamed A, Pekoz AY, Ross K, Hutcheon GA, Saleem IY. Pulmonary delivery of Nanocomposite Microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int J Pharm. 2019;569:118524. https://doi.org/10.1016/j.ijpharm.2019.118524.
Xu Y, Harinck L, Lokras AG, Gerde P, Selg E, Sjoberg CO, et al. Leucine improves the aerosol performance of dry powder inhaler formulations of siRNA-loaded nanoparticles. Int J Pharm. 2022;621:121758. https://doi.org/10.1016/j.ijpharm.2022.121758.
Kunda NK, Alfagih IM, Dennison SR, Tawfeek HM, Somavarapu S, Hutcheon GA, et al. Bovine serum albumin adsorbed PGA-co-PDL nanocarriers for vaccine delivery via dry powder inhalation. Pharm Res. 2015;32(4):1341–53. https://doi.org/10.1007/s11095-014-1538-5.
Kunda NK, Alfagih IM, Dennison SR, Somavarapu S, Merchant Z, Hutcheon GA, et al. Dry powder pulmonary delivery of cationic PGA-co-PDL nanoparticles with surface adsorbed model protein. Int J Pharm. 2015;492(1–2):213–22. https://doi.org/10.1016/j.ijpharm.2015.07.015.
Alfagih I, Kunda N, Alanazi F, Dennison SR, Somavarapu S, Hutcheon GA, et al. Pulmonary delivery of proteins using nanocomposite microcarriers. J Pharm Sci. 2015;104(12):4386–98. https://doi.org/10.1002/jps.24681.
Kwok PC, Tunsirikongkon A, Glover W, Chan HK. Formation of protein nano-matrix particles with controlled surface architecture for respiratory drug delivery. Pharm Res. 2011;28(4):788–96. https://doi.org/10.1007/s11095-010-0332-2.
Kunda NK, Alfagih IM, Miyaji EN, Figueiredo DB, Goncalves VM, Ferreira DM, et al. Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int J Pharm. 2015;495(2):903–12. https://doi.org/10.1016/j.ijpharm.2015.09.034.
Bielski E, Zhong Q, Mirza H, Brown M, Molla A, Carvajal T, et al. TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int J Pharm. 2017;527(1–2):171–83. https://doi.org/10.1016/j.ijpharm.2017.05.046.
Keil TW, Zimmermann C, Baldassi D, Adams F, Friess W, Mehta A, et al. Impact of crystalline and amorphous matrices on successful spray drying of siRNA polyplexes for inhalation of nano-in-microparticles. Adv Ther (Weinh). 2021;4(6). https://doi.org/10.1002/adtp.202100073.
Keil TWM, Feldmann DP, Costabile G, Zhong Q, da Rocha S, Merkel OM. Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. Eur J Pharm Biopharm. 2019;143:61–9. https://doi.org/10.1016/j.ejpb.2019.08.012.
Zimmermann CM, Baldassi D, Chan K, Adams NBP, Neumann A, Porras-Gonzalez DL, et al. Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J Control Release. 2022;351:137–50. https://doi.org/10.1016/j.jconrel.2022.09.021.
Gaspar DP, Serra C, Lino PR, Goncalves L, Taboada P, Remunan-Lopez C, et al. Microencapsulated SLN: an innovative strategy for pulmonary protein delivery. Int J Pharm. 2017;516(1–2):231–46. https://doi.org/10.1016/j.ijpharm.2016.11.037.
Nieto-Orellana A, Coghlan D, Rothery M, Falcone FH, Bosquillon C, Childerhouse N, et al. Dry-powder formulations of non-covalent protein complexes with linear or miktoarm copolymers for pulmonary delivery. Int J Pharm. 2018;540(1–2):78–88. https://doi.org/10.1016/j.ijpharm.2018.02.008.
Scherliess R, Janke J. Preparation of poly-lactic-co-glycolic acid nanoparticles in a dry powder formulation for pulmonary antigen delivery. Pharm. 2021;13(8). https://doi.org/10.3390/pharmaceutics13081196.
Qiu Y, Man RCH, Liao Q, Kung KLK, Chow MYT, Lam JKW. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release. 2019;314:102–15. https://doi.org/10.1016/j.jconrel.2019.10.026.
Wang JL, Hanafy MS, Xu H, Leal J, Zhai Y, Ghosh D, et al. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int J Pharm. 2021;596:120215. https://doi.org/10.1016/j.ijpharm.2021.120215.
Tran T-T, Amalina N, Cheow WS, Hadinoto K. Effects of storage on the stability and aerosolization efficiency of dry powder inhaler formulation of plasmid DNA-Chitosan nanoparticles. J Drug Deliv Sci Technol. 2020;59:101866. https://doi.org/10.1016/j.jddst.2020.101866.
Okuda T, Morishita M, Mizutani K, Shibayama A, Okazaki M, Okamoto H. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J Control Release. 2018;279:99–113. https://doi.org/10.1016/j.jconrel.2018.04.003.
Fukushige K, Tagami T, Naito M, Goto E, Hirai S, Hatayama N, et al. Developing spray-freeze-dried particles containing a hyaluronic acid-coated liposome-protamine-DNA complex for pulmonary inhalation. Int J Pharm. 2020;583:119338. https://doi.org/10.1016/j.ijpharm.2020.119338.
Zhang H, Zhang Y, Williams RO, 3rd HDC Smyth. Development of PEGylated chitosan/CRISPR-Cas9 dry powders for pulmonary delivery via thin-film freeze-drying. Int J Pharm. 2021;605:120831. https://doi.org/10.1016/j.ijpharm.2021.120831.
Xu PY, Kankala RK, Pan YJ, Yuan H, Wang SB, Chen AZ. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int J Nanomed. 2018;13:4685–98. https://doi.org/10.2147/IJN.S169399.
d’Angelo I, Conte C, Miro A, Quaglia F, Ungaro F. Pulmonary drug delivery: a role for polymeric nanoparticles? Curr Top Med Chem. 2015;15(4):386–400. https://doi.org/10.2174/1568026615666150108123256.
Elhissi A. Liposomes for pulmonary drug delivery: the role of formulation and inhalation device design. Curr Pharm Des. 2017;23(3):362–72. https://doi.org/10.2174/1381612823666161116114732.
Garcia-Fernandez A, Sancenon F, Martinez-Manez R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv Drug Deliv Rev. 2021;177:113953. https://doi.org/10.1016/j.addr.2021.113953.
Chan HW, Chow SF. Development of redispersible remdesivir nanoagglomerate for inhaled therapy: Box-Behnken design, optimization and in vitro aerosol performance. Montréal QC, Canada: Controlled Release Society 2022 Annual Meeting and Exposition; 2022.
Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, et al. L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm. 2016;102:132–41. https://doi.org/10.1016/j.ejpb.2016.02.010.
Scherließ R, Bock S, Bungert N, Neustock A, Valentin L. Particle engineering in dry powders for inhalation. Eur J Pharm Sci. 2022;172:106158. https://doi.org/10.1016/j.ejps.2022.106158.
Flume PA, Amelina E, Daines CL, Charlton B, Leadbetter J, Guasconi A, et al. Efficacy and safety of inhaled dry-powder mannitol in adults with cystic fibrosis: an international, randomized controlled study. J Cyst Fibros. 2021;20(6):1003–9. https://doi.org/10.1016/j.jcf.2021.02.011.
Flume PA, Aitken ML, Bilton D, Agent P, Charlton B, Forster E, et al. Optimising inhaled mannitol for cystic fibrosis in an adult population. Breathe (Sheff). 2015;11(1):39–48. https://doi.org/10.1183/20734735.021414.
Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci USA. 2002;99(19):12001–5. https://doi.org/10.1073/pnas.182233999.
Lee YY, Wu JX, Yang M, Young PM, van den Berg F, Rantanen J. Particle size dependence of polymorphism in spray-dried mannitol. Eur J Pharm Sci. 2011;44(1–2):41–8. https://doi.org/10.1016/j.ejps.2011.06.002.
Chow SF, Weng J, Xuan B, Wan KY. How can the challenges faced by nanoparticle-based pulmonary drug formulations be overcome. Ther Deliv. 2019;10(2):87–9. https://doi.org/10.4155/tde-2019-0001.
Larhrib H, Zeng XM, Martin GP, Marriott C, Pritchard J. The use of different grades of lactose as a carrier for aerosolised salbutamol sulphate. Int J Pharm. 1999;191(1):1–14. https://doi.org/10.1016/s0378-5173(99)00164-7.
Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24(3):411–37. https://doi.org/10.1007/s11095-006-9174-3.
Cheow WS, Li S, Hadinoto K. Spray drying formulation of hollow spherical aggregates of silica nanoparticles by experimental design. Chem Eng Res Des. 2010;88(5):673–85. https://doi.org/10.1016/j.cherd.2009.11.012.
Arpagaus C, Collenberg A, Rutti D, Assadpour E, Jafari SM. Nano spray drying for encapsulation of pharmaceuticals. Int J Pharm. 2018;546(1–2):194–214. https://doi.org/10.1016/j.ijpharm.2018.05.037.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83. https://doi.org/10.1208/s12248-014-9598-3.
Kho K, Cheow WS, Lie RH, Hadinoto K. Aqueous re-dispersibility of spray-dried antibiotic-loaded polycaprolactone nanoparticle aggregates for inhaled anti-biofilm therapy. Powder Technol. 2010;203(3):432–9. https://doi.org/10.1016/j.powtec.2010.06.003.
Malamatari M, Charisi A, Malamataris S, Kachrimanis K, Nikolakakis I. Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Process. 2020;8(7):788. https://doi.org/10.3390/pr8070788.
Yue PF, Li Y, Wan J, Yang M, Zhu WF, Wang CH. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int J Pharm. 2013;454(1):269–77. https://doi.org/10.1016/j.ijpharm.2013.06.050.
Gradon L, Sosnowski TR. Formation of particles for dry powder inhalers. Adv Powder Technol. 2014;25(1):43–55. https://doi.org/10.1016/j.apt.2013.09.012.
Zhu Q, Gou D, Li L, Chan H-K, Yang R. Numerical investigation of powder dispersion mechanisms in Turbuhaler and the contact electrification effect. Adv Powder Technol. 2022;33(11):103839. https://doi.org/10.1016/j.apt.2022.103839.
D’Addio SM, Chan JG, Kwok PC, Benson BR, Prud’homme RK, Chan HK. Aerosol delivery of nanoparticles in uniform mannitol carriers formulated by ultrasonic spray freeze drying. Pharm Res. 2013;30(11):2891–901. https://doi.org/10.1007/s11095-013-1120-6.
Innes E, Yiu HHP, McLean P, Brown W, Boyles M. Simulated biological fluids—a systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit Rev Toxicol. 2021;51(3):217–48. https://doi.org/10.1080/10408444.2021.1903386.
Jain P, Pawar RS, Pandey RS, Madan J, Pawar S, Lakshmi PK, et al. In-vitro in-vivo correlation (IVIVC) in nanomedicine: is protein corona the missing link? Biotechnol Adv. 2017;35(7):889–904. https://doi.org/10.1016/j.biotechadv.2017.08.003.
Weng J, Tong HHY, Chow SF. In vitro release study of the polymeric drug nanoparticles: development and validation of a novel method. Pharm. 2020;12(8). https://doi.org/10.3390/pharmaceutics12080732.
Wang W, Zhou QT, Sun SP, Denman JA, Gengenbach TR, Barraud N, et al. Effects of surface composition on the aerosolisation and dissolution of inhaled antibiotic combination powders consisting of colistin and rifampicin. AAPS J. 2016;18(2):372–84. https://doi.org/10.1208/s12248-015-9848-z.
Radivojev S, Luschin-Ebengreuth G, Pinto JT, Laggner P, Cavecchi A, Cesari N, et al. Impact of simulated lung fluid components on the solubility of inhaled drugs and predicted in vivo performance. Int J Pharm. 2021;606:120893. https://doi.org/10.1016/j.ijpharm.2021.120893.
Taki M, Marriott C, Zeng XM, Martin GP. Aerodynamic deposition of combination dry powder inhaler formulations in vitro: a comparison of three impactors. Int J Pharm. 2010;388(1–2):40–51. https://doi.org/10.1016/j.ijpharm.2009.12.031.
Clark AR, Weers JG, Dhand R. The confusing world of dry powder inhalers: it is all about inspiratory pressures, not inspiratory flow rates. J Aerosol Med Pulm Drug Deliv. 2020;33(1):1–11. https://doi.org/10.1089/jamp.2019.1556.
Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13. https://doi.org/10.1186/s40248-015-0012-5.
Weers J, Clark A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm Res. 2017;34(3):507–28. https://doi.org/10.1007/s11095-016-2050-x.
Malmberg LP, Everard ML, Haikarainen J, Lahelma S. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol Easyhaler((R)). J Aerosol Med Pulm Drug Deliv. 2014;27(5):329–40. https://doi.org/10.1089/jamp.2013.1099.
Faria-Urbina M, Ung KT, Lawler L, Zisman LS, Waxman AB. Inspiratory flow patterns with dry powder inhalers of low and medium flow resistance in patients with pulmonary arterial hypertension. Pulm Circ. 2021;11(2):20458940211012590. https://doi.org/10.1177/20458940211012591.
Haynes A, Geller D, Weers J, Ament B, Pavkov R, Malcolmson R, et al. Inhalation of tobramycin using simulated cystic fibrosis patient profiles. Pediatr Pulmonol. 2016;51(11):1159–67. https://doi.org/10.1002/ppul.23451.
Newman SP, Chan HK. In vitro-in vivo correlations (IVIVCs) of deposition for drugs given by oral inhalation. Adv Drug Deliv Rev. 2020;167:135–47. https://doi.org/10.1016/j.addr.2020.06.023.
Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8(17):4653–64. https://doi.org/10.1039/D0BM00558D.
Murday JS, Siegel RW, Stein J, Wright JF. Translational nanomedicine: status assessment and opportunities. Nanomed. 2009;5(3):251–73. https://doi.org/10.1016/j.nano.2009.06.001.
Feng J, Markwalter CE, Tian C, Armstrong M, Prud’homme RK. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J Transl Med. 2019;17(1):200. https://doi.org/10.1186/s12967-019-1945-9.
McNeil SE. Nanoparticle therapeutics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(3):264–71. https://doi.org/10.1002/wnan.6.
Tomoda K, Ohkoshi T, Kawai Y, Nishiwaki M, Nakajima T, Makino K. Preparation and properties of inhalable nanocomposite particles: effects of the temperature at a spray-dryer inlet upon the properties of particles. Colloids Surf B Biointerfaces. 2008;61(2):138–44. https://doi.org/10.1016/j.colsurfb.2007.07.016.
Pikal MJ, Pande P, Bogner R, Sane P, Mudhivarthi V, Sharma P. Impact of natural variations in freeze-drying parameters on product temperature history: application of quasi steady-state heat and mass transfer and simple statistics. AAPS PharmSciTech. 2018;19(7):2828–42. https://doi.org/10.1208/s12249-018-1155-4.
Kimura N, Maeki M, Sato Y, Note Y, Ishida A, Tani H, et al. Development of the iLiNP device: fine tuning the lipid nanoparticle size within 10 nm for drug delivery. ACS Omega. 2018;3(5):5044–51. https://doi.org/10.1021/acsomega.8b00341.
Bovone G, Steiner F, Guzzi EA, Tibbitt MW. Automated and continuous production of polymeric nanoparticles. Front Bioeng Biotechnol. 2019;7:423. https://doi.org/10.3389/fbioe.2019.00423.
Valsalakumari R, Yadava SK, Szwed M, Pandya AD, Maelandsmo GM, Torgersen ML, et al. Mechanism of cellular uptake and cytotoxicity of paclitaxel loaded lipid nanocapsules in breast cancer cells. Int J Pharm. 2021;597:120217. https://doi.org/10.1016/j.ijpharm.2021.120217.
Weng J, Shao Z, Chan HW, Li SPY, Lam JKW, Tsang CK, et al. Mediating bio-fate of polymeric cholecalciferol nanoparticles through rational size control. Biomater Adv. 2022;140:213074. https://doi.org/10.1016/j.bioadv.2022.213074.
Jeon S, Clavadetscher J, Lee DK, Chankeshwara SV, Bradley M, Cho WS. Surface charge-dependent cellular uptake of polystyrene nanoparticles. Nanomat (Basel). 2018;8(12). https://doi.org/10.3390/nano8121028.
Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater. 2012;24(28):3887–94. https://doi.org/10.1002/adma.201201800.
Ferreira AJ, Cemlyn-Jones J, Robalo CC. Nanoparticles, nanotechnology and pulmonary nanotoxicology. Rev Port Pneumol. 2013;19(1):28–37. https://doi.org/10.1016/j.rppneu.2012.09.003.
Bhavna Ahmad FJ, Mittal G, Jain GK, Malhotra G, Khar RK, et al. Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constrictive conditions. Eur J Pharm Biopharm. 2009;71(2):282–91. https://doi.org/10.1016/j.ejpb.2008.09.018.
Chow MYT, Tai W, Chang RYK, Chan HK, Kwok PCL. In vitro-in vivo correlation of cascade impactor data for orally inhaled pharmaceutical aerosols. Adv Drug Deliv Rev. 2021;177:113952. https://doi.org/10.1016/j.addr.2021.113952.
Lim SH, Park S, Lee CC, Ho PCL, Kwok PCL, Kang L. A 3D printed human upper respiratory tract model for particulate deposition profiling. Int J Pharm. 2021;597:120307. https://doi.org/10.1016/j.ijpharm.2021.120307.
Huang F, Zhu Q, Zhou X, Gou D, Yu J, Li R, et al. Role of CFD based in silico modelling in establishing an in vitro-in vivo correlation of aerosol deposition in the respiratory tract. Adv Drug Deliv Rev. 2021;170:369–85. https://doi.org/10.1016/j.addr.2020.09.007.
Sakagami M. In vitro, ex vivo and in vivo methods of lung absorption for inhaled drugs. Adv Drug Deliv Rev. 2020;161–162:63–74. https://doi.org/10.1016/j.addr.2020.07.025.
Park K. Questions on the role of the EPR effect in tumor targeting. J Control Release. 2013;172(1):391. https://doi.org/10.1016/j.jconrel.2013.10.001.
Funding
This work was financially supported by the Health and Medical Research Fund, Health Bureau, Hong Kong SAR Government (Project number: 08191956), and the Health@InnoHK program, Innovation and Technology Commission, Hong Kong SAR Government. HWC is an awardee of the Hong Kong PhD Fellowship Scheme, Research Grants Council, Hong Kong, and the HKU Presidential PhD Scholarship, The University of Hong Kong.
Author information
Authors and Affiliations
Contributions
HWC: conceptualization, investigation, writing—original draft, visualization. SC: investigation, writing—original draft, visualization. XZ: investigation, writing—original draft. YZ: visualization. HHYT: writing—review and editing. SFC: conceptualization, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Philip Kwok, Francesca Buttini, Jenny Lam and Vivek Gupta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chan, H.W., Chow, S., Zhang, X. et al. Inhalable Nanoparticle-based Dry Powder Formulations for Respiratory Diseases: Challenges and Strategies for Translational Research. AAPS PharmSciTech 24, 98 (2023). https://doi.org/10.1208/s12249-023-02559-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02559-y