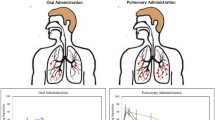
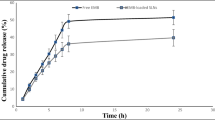
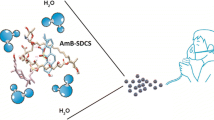
Abstract
Tuberculosis (TB) is a contiguous airborne disease caused by Mycobacterium tuberculosis (M.tb), primarily affecting the human lungs. The progression of drug-susceptible TB to drug-resistant strains, MDR-TB and XDR-TB, has become a global challenge toward eradicating TB. Conventional TB treatment involves frequent dosing and prolonged treatment regimens predominantly by an oral or invasive route, leading to treatment-related systemic adverse effects and patient’s noncompliance. Pulmonary delivery is an attractive option as we could reduce dose, limit systemic side-effects, and achieve rapid onset of action. Delamanid (DLD), an antituberculosis drug, has poor aqueous solubility, and in this study, we aim to improve its solubility using cyclodextrin complexation. We screened different cyclodextrins and found that HP-β-CD resulted in a 54-fold increase in solubility compared to a 27-fold and 13-fold increase by SBE-β-CD and HP-ɣ-CD, respectively. The stability constant (265 ± 15 M−1) and complexation efficiency (8.5 × 10−4) suggest the formation of a stable inclusion complex of DLD and HP-β-CD in a 2:1 ratio. Solid-state characterization studies (DSC, PXRD, and NMR) further confirmed successful complexation of DLD in HP-β-CD. The nebulized DLD-CD complex solution showed a mass median aerodynamic diameter of 4.42 ± 0.62 μm and fine particle fraction of 82.28 ± 2.79%, suggesting deposition in the respiratory airways. In bacterial studies, minimum inhibitory concentration of DLD-CD complex was significantly reduced (four-fold) compared to free DLD in M.tb (H37Ra strain). Furthermore, accelerated stability studies confirmed that the inclusion complex was stable for 4 weeks with 90%w/w drug content. In conclusion, we increased the aqueous solubility of DLD through cyclodextrin complexation and improved its efficacy in vitro.
Graphical Abstract








Similar content being viewed by others
References
Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global Tuberculosis Report 2020 – reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113:S7-12.
Global Pandemic [Internet]. TB Alliance. [cited 2022 Jul 1]. Available from: https://www.tballiance.org/why-new-tb-drugs/global-pandemic.
Natarajan A, Beena PM, Devnikar AV, Mali S. A systemic review on tuberculosis. Indian J Tuberc. 2020;67:295–311.
Pham D-D, Fattal E, Tsapis N. Pulmonary drug delivery systems for tuberculosis treatment. Int J Pharm. 2015;478:517–29.
Sotgiu G, Centis R, D’ambrosio L, Migliori GB. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med. 2015;5:a017822.
Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat Med. 2007;13:290–4.
Ausi Y, Santoso P, Sunjaya DK, Barliana MI. Between curing and torturing: burden of adverse reaction in drug-resistant tuberculosis therapy. Patient Prefer Adherence Dove Press. 2021;15:2597–607.
Padda IS, Reddy KM. Antitubercular medications [Internet]. StatPearls Internet. StatPearls Publishing; 2021 [cited 2022 Jul 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557666/.
The End TB Strategy [Internet]. [cited 2022 Jul 1]. Available from: https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy.
Ryan NJ, Lo JH. Delamanid: first global approval. Drugs. 2014;74:1041–5.
EMA. Deltyba [Internet]. Eur. Med. Agency. 2018 [cited 2022 Jul 1]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/deltyba.
Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J Eur Respir Soc. 2013;41:1393–400.
Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–60 (Massachusetts Medical Society).
Blair HA, Scott LJ. Delamanid: a review of its use in patients with multidrug-resistant tuberculosis. Drugs. 2015;75:91–100.
Stinson K, Kurepina N, Venter A, Fujiwara M, Kawasaki M, Timm J, et al. MIC of Delamanid (OPC-67683) against mycobacterium tuberculosis clinical isolates and a proposed critical concentration. Antimicrob Agents Chemother. 2016;60:3316–22 (American Society for Microbiology).
Xavier AS, Lakshmanan M. Delamanid: a new armor in combating drug-resistant tuberculosis. J Pharmacol Pharmacother. 2014;5:222–4.
Tao X, Gao C, Huang Z-G, Luo W, Liu K-L, Peng C-T, et al. Discovery and evaluation of novel nitrodihydroimidazooxazoles as promising anti-tuberculosis agents. Bioorg Med Chem Lett. 2019;29:2511–5.
Ramirez G, Pham AC, Clulow AJ, Salim M, Hawley A, Boyd BJ. Sustained absorption of delamanid from lipid-based formulations as a path to reduced frequency of administration. Drug Deliv Transl Res. 2021;11:1236–44.
Gupta D, Bhatia D, Dave V, Sutariya V, Varghese Gupta S. Salts of therapeutic agents: chemical, physicochemical, and biological considerations. Mol Basel Switz. 2018;23:1719 (MDPI).
Rautio J, Meanwell NA, Di L, Hageman MJ. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov. 2018;17:559–87.
Neslihan Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–82.
Salzano G, Wankar J, Ottani S, Villemagne B, Baulard AR, Willand N, et al. Cyclodextrin-based nanocarriers containing a synergic drug combination: a potential formulation for pulmonary administration of antitubercular drugs. Editor Spec Ed Int J Pharm Honor Profr Dominique Duchêne. 2017;531:577–87.
Arca HÇ, Mosquera-Giraldo LI, Pereira JM, Sriranganathan N, Taylor LS, Edgar KJ. Rifampin stability and solution concentration enhancement through amorphous solid dispersion in cellulose ω-carboxyalkanoate matrices. J Pharm Sci. 2018;107:127–38.
Gidwani B, Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res Int. 2015/10/25 ed. Hindawi Publishing Corporation; 2015;2015:198268–198268.
Haimhoffer Á, Rusznyák Á, Réti-Nagy K, Vasvári G, Váradi J, Vecsernyés M, et al. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci Pharm. 2019;87:33 (Multidisciplinary Digital Publishing Institute).
Sharma N, Baldi A. Exploring versatile applications of cyclodextrins: an overview. Drug Deliv Taylor & Francis. 2016;23:729–47.
Price DN, Kunda NK, Miller EK, Muttil P. Inhaled therapeutics against TB: the promise of pulmonary treatment and prevention strategies in the clinic. Inhal Aerosols. 3rd ed. CRC Press; 2019.
Mehta P, Bothiraja C, Kadam S, Pawar A. Potential of dry powder inhalers for tuberculosis therapy: facts, fidelity and future. Artif Cells Nanomed Biotechnol. 2018;46:S791-806 (Taylor & Francis).
Hanif SNM, Garcia-Contreras L. Pharmaceutical aerosols for the treatment and prevention of tuberculosis. Front Cell Infect Microbiol. 2012;2:118.
Braunstein M, Hickey AJ, Ekins S. Why wait? The case for treating tuberculosis with inhaled drugs. Pharm Res. 2019;36:166.
Parvathaneni V, Elbatanony RS, Goyal M, Chavan T, Vega N, Kolluru S, et al. Repurposing bedaquiline for effective non-small cell lung cancer (NSCLC) therapy as inhalable cyclodextrin-based molecular inclusion complexes. Int J Mol Sci. Multidisciplinary Digital Publishing Institute; 2021;22:4783 (1–18).
Sawant SS, Patil SM, Shukla SK, Kulkarni NS, Gupta V, Kunda NK. Pulmonary delivery of osimertinib liposomes for non-small cell lung cancer treatment: formulation development and in vitro evaluation. Drug Deliv Transl Res. 2021;1–14.
Patil SM, Sawant SS, Kunda NK. Inhalable bedaquiline-loaded cubosomes for the treatment of non-small cell lung cancer (NSCLC). Int J Pharm. 2021;607:121046 ((1-11)).
Vartak R, Patil SM, Saraswat A, Patki M, Kunda NK, Patel K. Aerosolized nanoliposomal carrier of remdesivir: an effective alternative for COVID-19 treatment in vitro. Nanomed Future Med. 2021;16:1187–202.
Patil SM, Kunda NK. Anticancer activity of D-LAK-120A, an antimicrobial peptide, in non-small cell lung cancer (NSCLC). Biochimie. 2022;201:7–17.
Ma DQ, Rajewski RA, Velde DV, Stella VJ. Comparative effects of (SBE)7m-β-CD and HP-β-CD on the stability of two anti-neoplastic agents, melphalan and carmustine. J Pharm Sci Elsevier. 2000;89:275–87.
Okimoto K, Rajewski RA, Uekama K, Jona JA, Stella VJ. The interaction of charged and uncharged drugs with neutral (HP-beta-CD) and anionically charged (SBE7-beta-CD) beta-cyclodextrins. Pharm Res. 1996;13:256–64.
Zia V, Rajewski RA, Stella VJ. Effect of cyclodextrin charge on complexation of neutral and charged substrates: comparison of (SBE)7M-β-CD to HP-β-CD. Pharm Res. 2001;18:667–73.
Vartiainen V, Bimbo LM, Hirvonen J, Kauppinen EI, Raula J. Aerosolization, drug permeation and cellular interaction of dry powder pulmonary formulations of corticosteroids with hydroxypropyl-β-cyclodextrin as a solubilizer. Pharm Res. 2017;34:25–35.
Mader WJ, Higuchi T. Phase solubility analysis. C R C Crit Rev Anal Chem. 1970;1:193–215 (Taylor & Francis).
Jambhekar SS, Breen P. Cyclodextrins in pharmaceutical formulations II: solubilization, binding constant, and complexation efficiency. Drug Discov Today. 2016;21:363–8.
Han D, Han Z, Liu L, Wang Y, Xin S, Zhang H, et al. Solubility enhancement of myricetin by inclusion complexation with heptakis-O-(2-hydroxypropyl)-β-cyclodextrin: a joint experimental and theoretical study. Int J Mol Sci. 2020;21:766 (Multidisciplinary Digital Publishing Institute).
Anjani QK, Domínguez-Robles J, Utomo E, Font M, Martínez-Ohárriz MC, Permana AD, et al. Inclusion complexes of rifampicin with native and derivatized cyclodextrins: in silico modeling, formulation, and characterization. Pharm Basel Switz. 2021;15:20.
Wu W, Xue W. Evaluation of anticancer activity of honokiol by complexation with hydroxypropyl-β-cyclodextrin. Colloids Surf B Biointerfaces. 2020;196:111298.
Vyas A, Gidwani B, Tripathi A, Dhurve P. Significance of jobs plot in cyclodextrin complexation. Res J Pharm Technol. 2016;9:1013–6 (Journal of Ravishankar University (Part-B)).
Ulatowski F, Dąbrowa K, Bałakier T, Jurczak J. Recognizing the limited applicability of job plots in studying host–guest interactions in supramolecular chemistry. J Org Chem Am Chem Soc. 2016;81:1746–56.
Sid D, Baitiche M, Elbahri Z, Djerboua F, Boutahala M, Bouaziz Z, et al. Solubility enhancement of mefenamic acid by inclusion complex with β-cyclodextrin: in silico modelling, formulation, characterisation, and in vitro studies. J Enzyme Inhib Med Chem. 2021;36:605–17 (Taylor & Francis).
Sbârcea L, Tănase I-M, Ledeți A, Cîrcioban D, Vlase G, Barvinschi P, et al. Encapsulation of risperidone by methylated β-cyclodextrins: physicochemical and molecular modeling studies. Molecules. 2020;25:5694 (Multidisciplinary Digital Publishing Institute).
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Mol Basel Switz. 2018;23:E1161.
Duong TV, Nguyen HT, Taylor LS. Combining enabling formulation strategies to generate supersaturated solutions of delamanid: in situ salt formation during amorphous solid dispersion fabrication for more robust release profiles. Eur J Pharm Biopharm. 2022;174:131–43.
Couto VM, de Oliveira-Nascimento L, Cabeça LF, Geraldes DC, Costa JSR, Riske KA, et al. Capsaicin-cyclodextrin complex enhances mepivacaine targeting and improves local anesthesia in inflamed tissues. Int J Mol Sci. 2020;21:5741 (Multidisciplinary Digital Publishing Institute).
Giordano F, Novak C, Moyano JR. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim Acta. 2001;380:123–51.
Hao X, Sun X, Zhu H, Xie L, Wang X, Jiang N, et al. Hydroxypropyl-β-cyclodextrin-complexed resveratrol enhanced antitumor activity in a cervical cancer model: in vivo analysis. Front Pharmacol [Internet]. 2021 [cited 2022 Jul 10];12. Available from: https://www.frontiersin.org/articles/10.3389/fphar.2021.573909.
Figueiras A, Cardoso O, Veiga F, Carvalho RB, Ballaro G. Preparation and characterization of trimethoprim inclusion complex with methyl-β-cyclodextrin and determination of its antimicrobial activity [Internet]. 2015 [cited 2022 Jul 10]. Available from: https://www.semanticscholar.org/paper/Preparation-and-characterization-of-Trimethoprim-of-Figueiras-Cardoso/96a6cf9f374d1aa8b8d7286101b139fe90caf28a.
Zheng Y, Chow AHL. Production and characterization of a spray-dried hydroxypropyl-β-cyclodextrin/quercetin complex. Drug Dev Ind Pharm. 2009;35:727–34 (Taylor & Francis).
Cyclodextrin: a promising candidate in enhancing oral bioavailability of poorly water soluble drugs. MOJ Bioequivalence Bioavailab [Internet]. MedCrave Publishing; 2017 [cited 2022 Jul 10];Volume 3. Available from: https://medcraveonline.com/MOJBB/MOJBB-03-00034.pdf.
Muthu MS, Feng S-S. Pharmaceutical stability aspects of nanomedicines. Nanomed Future Med. 2009;4:857–60.
Jacob S, Nair AB. Cyclodextrin complexes: perspective from drug delivery and formulation. Drug Dev Res. 2018;79:201–17.
Wang X, Parvathaneni V, Shukla SK, Kulkarni NS, Muth A, Kunda NK, et al. Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int J Biol Macromol. 2020;164:638–50.
Rosati JA, Leith D, Kim CS. Monodisperse and polydisperse aerosol deposition in a packed bed. Aerosol Sci Technol. 2003;37:528–35.
Guan M, Zeng X, Shi R, Zheng Y, Fan W, Su W. Aerosolization performance, antitussive effect and local toxicity of naringenin-hydroxypropyl-β-cyclodextrin inhalation solution for pulmonary delivery. AAPS PharmSciTech. 2021;22:20.
Su W, Liang Y, Meng Z, Chen X, Lu M, Han X, et al. Inhalation of tetrandrine-hydroxypropyl-β-cyclodextrin inclusion complexes for pulmonary fibrosis treatment. Mol Pharm Am Chem Soc. 2020;17:1596–607.
Evrard B, Bertholet P, Gueders M, Flament M-P, Piel G, Delattre L, et al. Cyclodextrins as a potential carrier in drug nebulization. J Control Release Off J Control Release Soc. 2004;96:403–10.
Adhikari BR, Dummer J, Gordon KC, Das SC. An expert opinion on respiratory delivery of high dose powders for lung infections. Expert Opin Drug Deliv. 2022;0:1–19 (Taylor & Francis).
Shibata M, Shimokawa Y, Sasahara K, Yoda N, Sasabe H, Suzuki M, et al. Absorption, distribution and excretion of the anti-tuberculosis drug delamanid in rats: extensive tissue distribution suggests potential therapeutic value for extrapulmonary tuberculosis. Biopharm Drug Dispos. 2017;38:301–12.
Buttini F, Colombo G. Formulation strategies for antitubercular drugs by inhalation. Drug Deliv Syst Tuberc Prev Treat [Internet]. John Wiley & Sons, Ltd; 2016 [cited 2022 Jul 11]. p. 197–212. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118943182.ch10.
He D, Deng P, Yang L, Tan Q, Liu J, Yang M, et al. Molecular encapsulation of rifampicin as an inclusion complex of hydroxypropyl-β-cyclodextrin: design; characterization and in vitro dissolution. Colloids Surf B Biointerfaces. 2013;103:580–5.
Tewes F, Brillault J, Couet W, Olivier J-C. Formulation of rifampicin-cyclodextrin complexes for lung nebulization. J Control Release Off J Control Release Soc. 2008;129:93–9.
Amaro BR, Alves CCS, Ferreira GF, Carvalho PE, da Silva JG, Souza CA, et al. Multifunctionality of βCD/ofloxacin and HPβCD/ofloxacin complexes: improvement of the antimicrobial activity and apoptosis induction on lung adenocarcinoma A549 cells. J Braz Chem Soc. 2020;31:2628–37 (Sociedade Brasileira de Química).
Salem II, Steffan G, Düzgünes N. Efficacy of clofazimine–modified cyclodextrin against Mycobacterium avium complex in human macrophages. Int J Pharm. 2003;260:105–14.
Funding
This study was supported by funds provided to NKK by the Department of Pharmaceutical Sciences and College of Pharmacy and Health Sciences (CPHS), St. John’s University. SMP, DSB, TC, KP, and VP were supported by teaching assistantship from the Department of Pharmaceutical Sciences, CPHS, St. John’s University.
Author information
Authors and Affiliations
Contributions
Conceptualization: SMP, DSB, NKK; methodology: SMP, DSB, TC, KP, VP, AM, NKK; formal analysis: SMP, DSB, TC, KP, VP, AM; investigation: SMP, DSB, TC, KP, VP, AM; data curation: SMP, DSB, TC, KP, AJC, VP, AM, NKK; writing — original draft preparation: SMP, DSB, TC, KP, VP, AM, NKK; writing — review and editing: SMP, KP, AM, NKK; resources: NKK; visualization: SMP, KP, AM, NKK; supervision: AM, NKK; project administration: NKK; funding acquisition: NKK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suyash M. Patil and Druva Sarika Barji are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, S.M., Barji, D.S., Chavan, T. et al. Solubility Enhancement and Inhalation Delivery of Cyclodextrin-Based Inclusion Complex of Delamanid for Pulmonary Tuberculosis Treatment. AAPS PharmSciTech 24, 49 (2023). https://doi.org/10.1208/s12249-023-02510-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02510-1