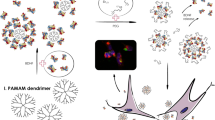
Abstract
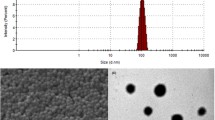
Piperine (PIP) is a neuroprotective phytomedicine that has profound acetylcholine esterase and reactive oxygen species inhibition effect in Alzheimer’s disease (AD) model. However, the oral delivery of PIP is limited by poor aqueous solubility and low bioavailability in systemic circulation. To improve the PIP bioavailability, the polyamidoamine (PAMAM) G4 dendrimer is grafted with tocopheryl polyethylene glycol succinate-1000 (TPGS) through carbodiimide chemistry to form TPGS-PAMAM conjugate. The TPGS-PAMAM coupling was confirmed through proton NMR and FTIR techniques. PIP was encapsulated in the TPGS-PAMAM through solvent diffusion method to form PIP-TPGS-PAMAM. The particle size for PIP-TPGS-PAMAM found the less than 50 nm, whereas entrapment efficiency found to 87 ± 3.5% and 10.6 ± 2.9% drug loading. The powder differential scanning calorimetry and powder X-ray diffraction characterization were employed to evaluate the amorphous encapsulation of the PIP in TPGS-PAMAM. The PIP-TPGS-PAMAM stability was studied in the gastric fluids which showed no drastic difference in particle size and encapsulation efficiency compared to PIP-PAMAM. The in vitro release analysis revealed 37 ± 4.1% PIP release from the PIP-TPGS-PAMAM matrix, and 71 ± 4.9% PIP release from the PIP-PAMAM dendrimer was observed in 48 h. The single-dose oral gavage to Wistar rats of PIP-TPGS-PAMAM showed the AUC0-∞ 14.38 µg/mL.h, Cmax 7.77 ± 1.65 µg/mL, Tmax, 1.6 ± 0.18 h, and half-life 3.47 ± 0.64 h for PIP in systemic circulation. PIP-PAMAM and free PIP showed significantly poor AUC0-∞ compared to PIP-TPGS-PAMAM. The brain uptake studies revealed PIP-TPGS-PAMAM treated group showed 2.2 ± 0.37 µg/g PIP content compared to free PIP administered group which was 0.4 ± 0.10 µg/g. Therefore, PIP-TPGS-PAMAM can offer excellent prospect for the delivery hydrophobic drugs to brain in AD.
Graphical Abstract









Similar content being viewed by others
Data Availability
All experimental or analysis data of this study are reported in manuscript tables and figures.
References
Brambilla D, le Droumaguet B, Nicolas J, Hashemi SH, Wu LP, Moghimi SM, et al. Nanotechnologies for Alzheimer’s disease: diagnosis, therapy, and safety issues. Nanomedicine: Nanotechnology. Biol Med Elsevier. 2011;7:521–40.
Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? Pharmacy and Therapeutics. 2010;35:208–11.
Henry W, Querfurth HW, LaFerla FM. Mechanisms of disease Alzheimer’s disease. New Engl J Med. 2010;362:329–44.
Chonpathompikunlert P, Wattanathorn J, Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem Toxicol Pergamon. 2010;48:798–802.
Elnaggar YSR, Etman SM, Abdelmonsif DA, Abdallah OY. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci Elsevier. 2015;104:3544–56.
Etman SM, Elnaggar YSR, Abdelmonsif DA, Abdallah OY. Oral brain-targeted microemulsion for enhanced piperine delivery in Alzheimer’s disease therapy: in vitro appraisal, in vivo activity, and nanotoxicity. AAPS PharmSciTech. 2018;19:3698–711.
Handa M, Tiwari S, Yadav AK, Almalki WH, Alghamdi S, Alharbi KS, et al. Therapeutic potential of nanoemulsions as feasible wagons for targeting Alzheimer’s disease. Drug Discov Today Elsevier Current Trends. 2021;26:2881–8.
Shukla R, Singh A, Handa M, Flora SJS, Kesharwani P. Nanotechnological approaches for targeting amyloid-β aggregation with potential for neurodegenerative disease therapy and diagnosis. Drug Discov Today Elsevier Current Trends. 2021;26:1972–9.
Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Future Medicine Ltd London, UK. 2009;4:557–74. Available from: https://www.futuremedicine.com/doi/abs/10.2217/nnm.09.38
Ray E, Vaghasiya K, Sharma A, Shukla R, Khan R, Kumar A, Verma RK. Autophagy-inducing inhalable co-crystal formulation of niclosamide-nicotinamide for lung cancer therapy. AAPS PharmSciTech. 2020;21:1–14. Available from: https://link.springer.com/article/10.1208/s12249-020-01803-z
Khairnar P, Handa M, Shukla R. Nanocrystals: an approachable delivery system for anticancer therapeutics. Current Drug Metabolism Bentham Science Publishers Ltd. 2022;23.
Mule S, Khairnar P, Shukla R. Recent advances in nanocrystals heralding greater potential in brain delivery. Particle & Particle Systems Characterization John Wiley & Sons, Ltd. 2022;2200087. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/ppsc.202200087
Xu L, Zhang H, Wu Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem Neurosci. 2014;5:2–13. Available from: https://pubs.acs.org/doi/abs/10.1021/cn400182z
Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev Elsevier. 2005;57:2215–37.
Santos SD, Xavier M, Leite DM, Moreira DA, Custódio B, Torrado M, et al. PAMAM dendrimers: blood-brain barrier transport and neuronal uptake after focal brain ischemia. J Control Release Elsevier. 2018;291:65–79.
Guo Y, Chu M, Tan S, Zhao S, Liu H, Otieno BO, Yang X, Xu C, Zhang Z. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol Pharm. 2014;11:59–70. Available from: https://pubs.acs.org/doi/abs/10.1021/mp400514t
Singh A, Ujjwal RR, Naqvi S, Verma RK, Tiwari S, Kesharwani P, Shukla R. Formulation development of tocopherol polyethylene glycol nanoengineered polyamidoamine dendrimer for neuroprotection and treatment of Alzheimer disease. J Drug Target. 2022;7:1–52. Available from: https://www.tandfonline.com/doi/abs/10.1080/1061186X20222063297
Örberg ML, Schillén K, Nylander T. Dynamic light scattering and fluorescence study of the interaction between double-stranded DNA and poly(amido amine) dendrimers. Biomacromolecules. 2007;8:1557–63. Available from: https://pubs.acs.org/doi/abs/10.1021/bm061194z
Tawfik MA, Tadros MI, Mohamed MI. Polyamidoamine (PAMAM) dendrimers as potential release modulators and oral bioavailability enhancers of vardenafil hydrochloride. Pharm Dev Technol. 2018;24:293–302. Available from: https://www.tandfonline.com/doi/abs/10.1080/10837450.2018.1472611
Pooja D, Kulhari H, Singh MK, Mukherjee S, Rachamalla SS, Sistla R. Dendrimer–TPGS mixed micelles for enhanced solubility and cellular toxicity of taxanes. Colloids Surf B: Biointerfaces Elsevier. 2014;121:461–8.
Gardikis K, Hatziantoniou S, Viras K, Wagner M, Demetzos C. A DSC and Raman spectroscopy study on the effect of PAMAM dendrimer on DPPC model lipid membranes. Int J Pharm Elsevier. 2006;318:118–23.
Tajabadi M, Khosroshahi ME, Bonakdar S. An efficient method of SPION synthesis coated with third generation PAMAM dendrimer. . Colloids Surf A: Physicochem Eng Aspects Elsevier. 2013;431:18–26.
Jackson CL, Chanzy HD, Booy FP, Drake BJ, Tomalia DA, Bauer BJ, Amis EJ. Visualization of dendrimer molecules by transmission electron microscopy (TEM): staining methods and cryo-TEM of vitrified solutions. Macromolecules. 1998;31:6259–65. Available from: https://pubs.acs.org/doi/abs/10.1021/ma9806155
Krishna KV, Wadhwa G, Alexander A, Kanojia N, Saha RN, Kukreti R, et al. Design and biological evaluation of lipoprotein-based donepezil nanocarrier for enhanced brain uptake through oral delivery. ACS Chem Neurosci Am Chem Soc. 2019;10:4124–35.
Chauhan AS, Sridevi S, Chalasani KB, Jain AK, Jain SK, Jain NK, et al. Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin. J Control Release Elsevier. 2003;90:335–43.
Liu J, Bi Y, Luo R, Wu X. Simultaneous UFLC–ESI–MS/MS determination of piperine and piperlonguminine in rat plasma after oral administration of alkaloids from Piper longum L.: application to pharmacokinetic studies in rats. J Chromatography B Elsevier. 2011;879:2885–90.
Asthana A, Chauhan AS, Diwan PV, Jain NK. Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site specific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech. 2005;6:536–42. Available from: https://link.springer.com/article/10.1208/pt060367
He H, Li Y, Jia XR, Du J, Ying X, Lu WL, et al. PEGylated poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials Elsevier. 2011;32:478–87.
Khare V, Sakarchi W al, Gupta PN, Curtis ADM, Hoskins C. Synthesis and characterization of TPGS–gemcitabine prodrug micelles for pancreatic cancer therapy. RSC Advances. 2016;6:60126–37. Available from: https://pubs.rsc.org/en/content/articlehtml/2016/ra/c6ra09347g
Perez M. Gibbs-Thomson effects in phase transformations. Scripta Materialia Pergamon. 2005;52:709–12.
Fox LJ, Richardson RM, Briscoe WH. PAMAM dendrimer - cell membrane interactions. Adva Colloid Interface Sci Elsevier. 2018;257:1–18.
Won YW, Yoon SM, Sonn CH, Lee KM, Kim YH. Nano self-assembly of recombinant human gelatin conjugated with α-tocopheryl succinate for Hsp90 inhibitor, 17-AAG, delivery. ACS Nano. 2011;5:3839–48. Available from: https://pubs.acs.org/doi/abs/10.1021/nn200173u
Chen W, Miao YQ, Fan DJ, Yang SS, Lin X, Meng LK, Tang X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12:705–11. Available from: https://link.springer.com/article/10.1208/s12249-011-9632-z
Meng X, Liu J, Yu X, Li J, Lu X, Shen T. Pluronic F127 and D-α-tocopheryl polyethylene glycol succinate (TPGS) mixed micelles for targeting drug delivery across the blood brain barrier. Sci Rep. 2017;7:1–12. Available from: https://www.nature.com/articles/s41598-017-03123-y
Kadam RS, Bourne DWA, Kompella UB. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: contribution of reduced clearance. Drug Metab Dispos. 2012;40:1380–8. Available from: https://dmd.aspetjournals.org/content/40/7/1380
Acknowledgements
The authors acknowledge the Department of Pharmaceuticals under the Ministry of Chemicals and Fertilizers, Government of India, for research facility. We also acknowledge the Animal Ethics Committee of NIPER-R for allowing the animal experiments having no. NIPER/RBL/IAEC/80/August 2021. The NIIPER communication no. is 317.
Author information
Authors and Affiliations
Contributions
Dr. Rahul Shukla, supervision, conceptualization, methodology, and investigation. Ajit Singh, validation, visualization, data curation, analysis, data interpretation, and investigation. Akshada Mhaske, review and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, A., Mhaske, A. & Shukla, R. Fabrication of TPGS-Grafted Polyamidoamine Dendrimer for Enhanced Piperine Brain Delivery and Pharmacokinetics. AAPS PharmSciTech 23, 236 (2022). https://doi.org/10.1208/s12249-022-02393-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02393-8